Guest Post by Jeremy Pasatta, Advanced Polymer Coatings
Anhydride curing agents for epoxies are a popular choice amongst formulators because they give the final cured formulation very high thermal properties along with excellent electrical properties, and their high use level and low viscosity allow them to be used in applications requiring this low viscosity, such as potting, filament winding and other composite production techniques. These curing agents typically require heat to cure in reasonable amounts of time for commercial production parts, and much like other latent curing agents for epoxies, the use of accelerators can reduce the time and curing temperature to maximize cycle time and throughput.
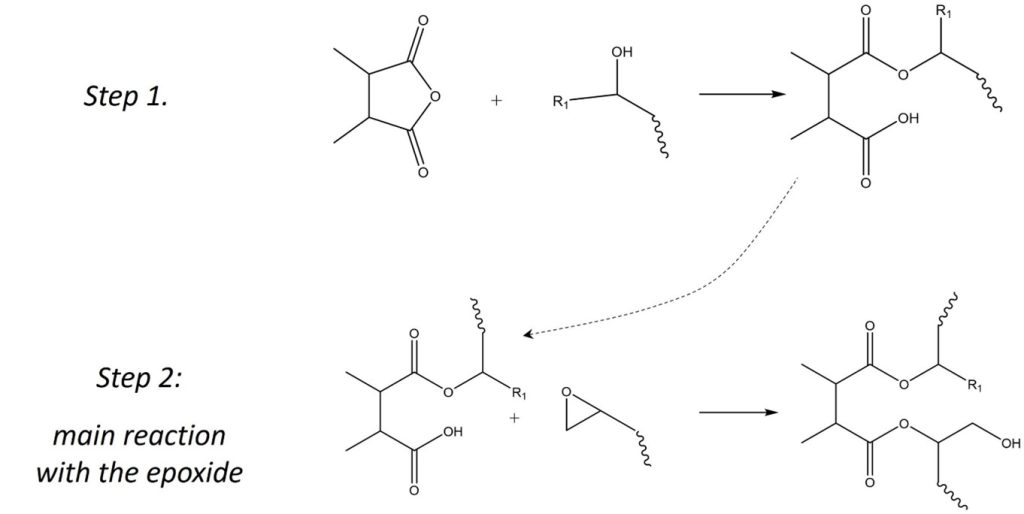
In order to understand what type of accelerators are used for anhydrides it’s important first to look at the general curing mechanism for anhydrides, which is shown in Figure 1. The anhydride ring is first opened by a hydroxyl or carboxylic acid, which can be present in the formulation as trace amounts of water, can come from hydroxyl groups present on the epoxy resin or from trace amounts of residual catalyst from the manufacture of the epoxy resin. In this way, the reaction between epoxy and anhydrides already comes with small amounts of an internal accelerator.
Figure 1. Anhydride Epoxy Curing Reaction
But what if this accelerating effect is not enough? Below are several other types of accelerators that can be added for the epoxy reaction with anhydrides.
- Tertiary amines
- Imidizoles
- Urea accelerators
- Alcohols
- Carboxylic acids
- Quaternary ammonium salts
- Organometallic salts
What these accelerators all have in common is that they open the epoxy ring either through direct reaction with the anhydride or through promoting homopolymerization of the epoxy, which in turn produces secondary hydroxyl groups on the epoxy resin which then opens the anhydride ring.
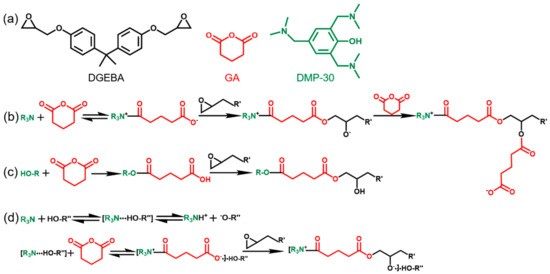
One of the more common accelerators for epoxy is 2,4,6-tris(dimethylaminomethyl)phenol (DMP-30, MW: 265.39 g/mol). Figure 2 shows 2 phenyl 4-methyl imidazole. The reaction between bisphenol A based epoxy and glutaric acid accelerated with DMP-30.
Figure 2. Acceleration of Epoxy-Anhydride Reaction with DMP-30 [SOURCE: Polymers 2021, 13(2), 296; https://doi.org/10.3390/polym13020296]
DMP-30 is unique as an accelerator for anhydrides because there are several different mechanisms by which the anhydride ring can be opened as shown in Figure 2. Step B shows the anhydride ring opening by the tertiary amine, Step C shows the mechanism for ring opening by hydroxyl group, and Step D shows the ring opening through the interaction of the tertiary amine/non-aromatic hydroxy complex and the anhydride. What is common to all these ring opening mechanisms is the ring is opened and secondary hydroxyls are formed on the epoxy, which allows for further reaction with the anhydride.
The typical weight percentage of accelerators used in epoxy anhydride systems are in the range of 1 to 5 percent. For instance, in the study shown in Figure 2, the weight percentage of DMP-30 was 2 percent, while the stoichiometry of the epoxy to acyl group in the glutaric anhydride ranged from 1:1 to 1:2. The choice of accelerator can also affect the color of the final formulation. Tertiary amines and imidizoles can impart amber or brown color to the formulation, whereas organometallic salts such as benzyltriethylamonium chloride (BTEAC) dissolved in ethylene glycol can result in final formulations that are very low in color.
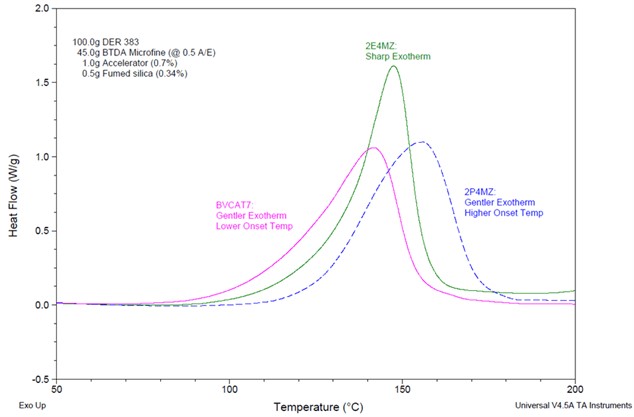
The choice of accelerator for anhydride epoxy curing reactions can not only affect the time and temperature required to cure, but can also effect the degree of snap cure or produce a more gentle cure. Figure 3 shows the effect of three different catalysts, two imidizoles (2E4MZ (2-ethyl-4-methylimidazole) and 2P4MZ (2-phenyl-4-methylimidazole)) and one quaternary ammonium salt (BVCAT7).
Figure 3. Effect of Accelerator Type on Exotherm [SOURCE: https://www.azom.com/article.aspx?ArticleID=20902]
While the 2E4MZ gives a very sharp exotherm which would equate to a snap cure, depending on the part size this may give localized hot spots which could result in some areas of weakness in the final cured part. The other two parts give a gentler exotherm which may be desirable for some processes, and the quaternary ammonium salt gave a peak exotherm of about 145˚C compared to a peak exotherm of about 160˚C for the 2P4MZ.
This blog post has shown that there are a wide variety of accelerators for the epoxy anhydride reaction, and that proper choice will often depend on not only the properties of the final cured formulation but also the processing technique and curing conditions required based on the part geometry and thickness. It’s extremely important for formulators to work closely with supplier partners for the correct selection of accelerators in order to optimize properties and processing. In the next blog post we’ll dive into accelerators for amine cured epoxies, which is an equally complex and interesting topic.

Sir can you explain what is the basic mechanism is between the various phenol compounds DMP 30 , Nonylphenol, and even raw phenol, that accelerate the Epoxy resin Amine cure reaction as they do?
The mechanism was explained in a previous post on “Accelerators for Amine Cured Epoxies” published on February 13, 2023.