Guest Post by Jeremy Pasatta, Huntsman Corporation
This is the first in a series of six guest posts by Jeremy Pasatta.
Curing agents for epoxies are one of the most critical, and often overlooked, parts of a formulation. The correct choice of curing agent can dramatically improve the properties of the formulation such as heat resistance and flexibility while also allowing curing at lower temperatures for example. Perhaps the largest category of curing agents for epoxy resins are amines. In part 1 of this series of blog posts, we’ll take a look at how amines react with epoxy resins, along with the general categories of amines and their relative strengths and weaknesses. In Part 2, we’ll take a deeper look into how the correct choice of amine can really enhance formulation properties.
The chemical structure of amines is characterized by a nitrogen that can contain either two, one or no hydrogens. This is critical to how the amine interacts with the epoxy, because it is the hydrogen on the amine molecule that is reactive to the epoxy resin, while an amine with no hydrogens can act as an accelerator but does not react with the epoxy. These amine types are summarized below:
- Primary amine (1°) – Two active hydrogens, most reactive with epoxy resins
- Secondary amine (2°) – One active hydrogen, 2x less reactive than a primary amine
- Tertiary amine (3°) – non-reactive with epoxy resins, but can accelerate curing reactions
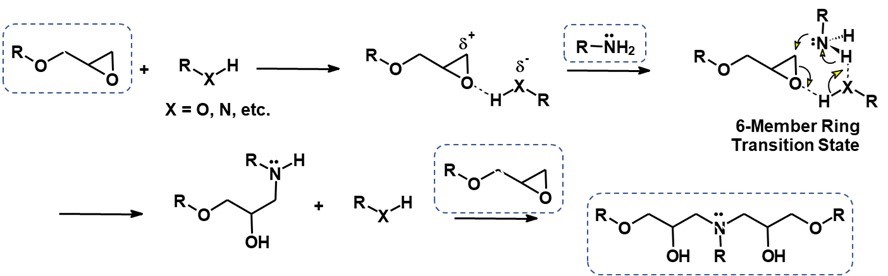
The hydrogen of the amine attacks the epoxy ring and undergoes the reaction mechanism shown in Figure 1.
Figure 1. Reaction Mechanism of Amine and Epoxy
For a more detailed discussion of the epoxy-amine reaction chemistry, visit a previous post called Epoxy Cure Chemistry Part 4: Nucleophiles in Action
When formulators are working with amines one of the most critical values is the number of hydrogens that are available to react with the epoxy. Suppliers of amine curing agents will typically list this value on a certificate of analysis as Amine Hydrogen Equivalent Weight, or AHEW. To calculate how many grams of amine curing agent would be required to cure 100 grams of epoxy resin, which is known as PHR, the formula shown in Figure 2 is used.
Figure 2. Equation used to calculate the amine PHR
Often in formulations a blend of multiple epoxy resins or multiple amine curing agents are used to achieve the desired end properties or to achieve a lower viscosity for example. To calculate the EEW of an epoxy blend or AHEW of an amine blend, a weighted average is used, which is given in Figure 3. The weighted average is just the sum of the individual component weights times the weight percent of that component in the blend. These weighted averages can then be used in Figure 2 to calculate the PHR of amine curing agent.
Figure 3. Weighted average calculation for epoxy or amine blends
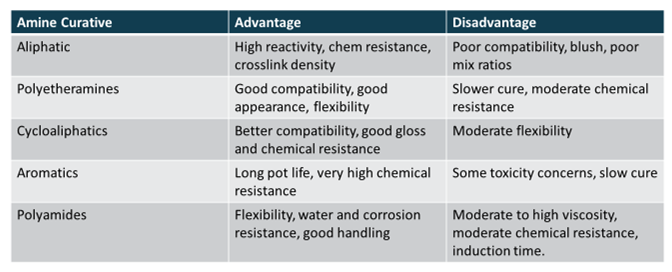
A huge variety of chemical structures can be attached to the amine. Chemists have come up with a wide variety of structures of amines that can be used as curing agents for epoxies, each with its own unique strengths and weaknesses depending on the structure. Some of the most common types of amine structures are listed in Table 1, along with their relative advantages and disadvantages.
Table 1. Common Amine Curing Agent Types.
It becomes very evident from the information in Table 1 that there is no perfect amine curing agent. Based on this information, a formulator must carefully weight the advantages and disadvantages of each curing agent to achieve whatever their goal is for their formulation. A balance of properties can also be achieved by blending different types of amine curing agents. For instance, for slower curing amines, aliphatic amines can be blended at small amount to speed up the cure time while still retaining the properties of the main curing agent.
Part 1 of this blog series has given a general introduction to amines, the largest class of curing agents for epoxy resins. We have a general understanding of how amines work and how to calculate the amount of amine required to cure an epoxy resin. In the next post, Part 2 of the blog series on epoxy curing agents, we’ll take a closer look at the structure property relationships of amines and how to pick the right amine to bring unique properties to epoxy formulations.


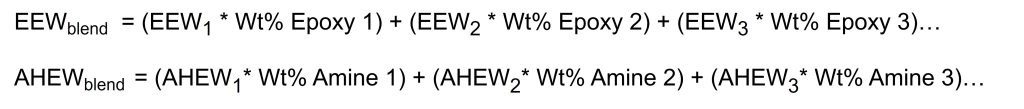
Beautifully explained the basics of Amine and Epoxy
Consider adding an option to adjust for resin shrinkage, as different resins may reduce in volume slightly after curing.