The next few posts will discuss other types of thermosetting polymers including vinyl esters, unsaturated polyesters, and polyurethanes.
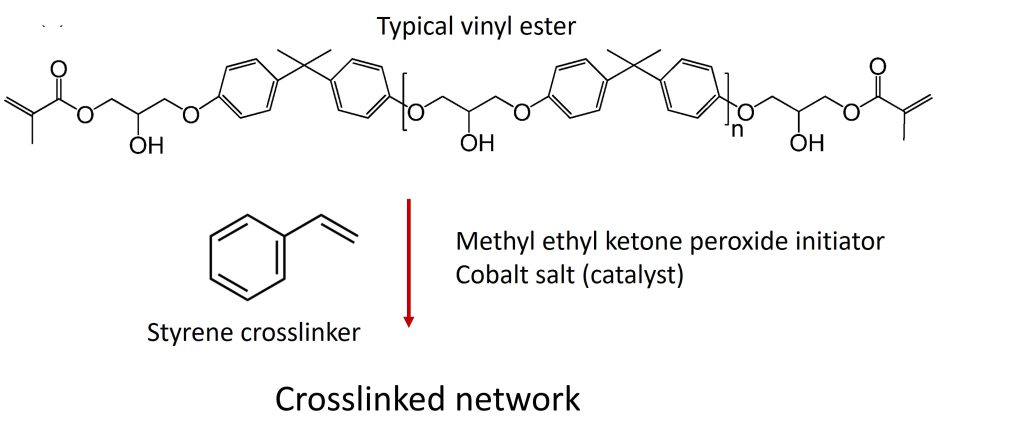
Vinyl esters are a class of thermosetting polymers that have properties intermediate between unsaturated polyester and epoxy. Vinyl esters are cured in a similar manner to unsaturated polyester in that the vinyl esters are combined with styrenic monomers (most commonly styrene) to form crosslinked networks.
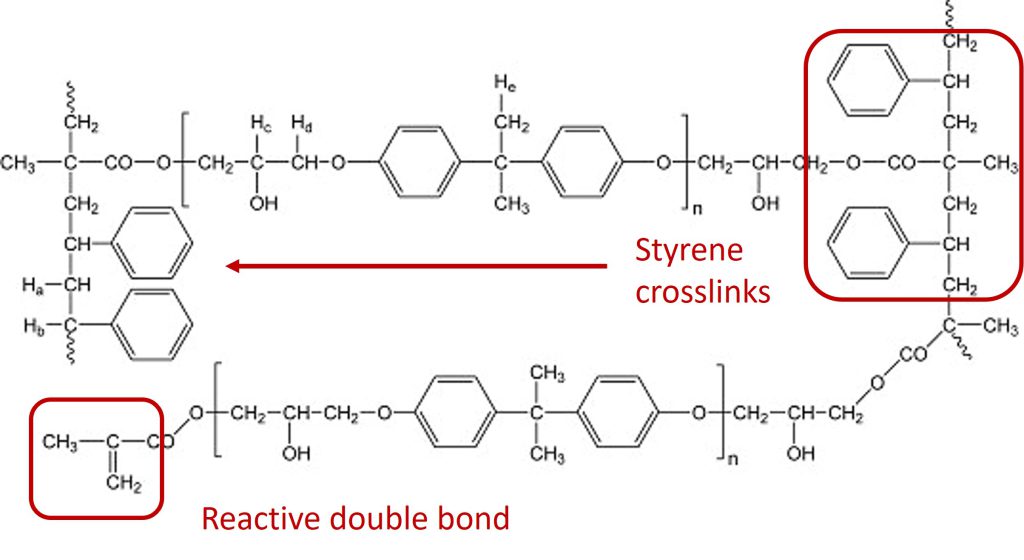
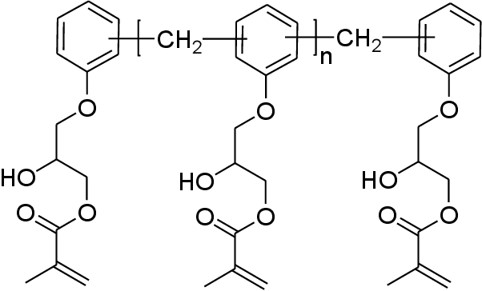
In the case of vinyl esters cured with styrene, the free radicals attack the double bonds at the ends of the vinyl ester molecule creating the crosslinks between the segments with the aromatic rings leading to the following structure:
Schematic of a cured network formed by the reaction of vinyl ester with styrene monomers.
The styrene free radical reaction occurs at the ends of the vinyl ester molecule as shown in red. The reactive double bond is also highlighted at the end of the vinyl ester molecule in the lower left.
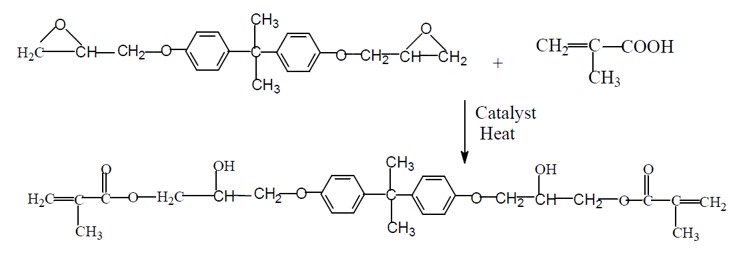
Vinyl esters are synthesized by reacting diglycidyl ether of bisphenol A with acrylic acid or methacrylic acid as shown below. Vinyl esters incorporate aromatic rings into the backbone along with flexible aliphatic segments.
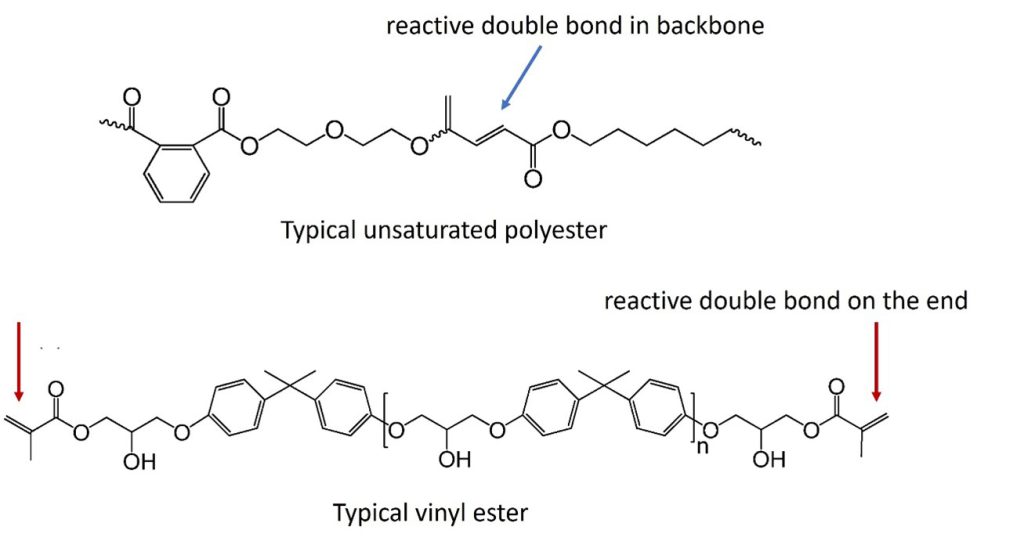
Catalysts for the epoxide – acrylic acid reaction are ammonium salts, tertiary amines and phosphines and the reaction occurs at moderately elevated temperatures [1]. The vinyl ester monomer has double bonds on each end of the chain. Examining the chemical structures of a typical unsaturated polyester and a vinyl ester shows the locations of the double bonds. In the case of the unsaturated polyester, the reactive double bond is in the backbone. In the case of the vinyl ester, the reactive double bond is at the end of the molecule.
Vinyl esters may also be synthesized using epoxy novolac and acrylic and methacrylic acids leading to a vinyl ester shown below.
Epoxy novolac-based vinyl esters provide cured networks with higher heat deflection temperatures (glass transition) while maintaining excellent chemical resistance.
Vinyl ester double bonds are polymerized using free radical initiators such as peroxides and through photopolymerization. Vinyl esters can be homopolymerized or alternately copolymerized by dissolving the vinyl ester monomer or oligomers into unsaturated reactive monomers such as styrene and vinyl toluene forming liquid resins that may be processed much like unsaturated polyesters.
The crosslink density is typically lower for cured vinyl ester networks since there are fewer C=C double bonds in the starting structure. With that said, the more rigid backbone from the diglycidyl ether of bisphenol A results in increased toughness, improved impact strength and greater tensile elongation at similar Youngs Modulus when compared with unsaturated polyester networks [1].
Cured vinyl ester networks exhibit improved chemical resistance compared to unsaturated polyester networks which is due in part to the absence of ester linkages in the epoxy backbone portion where the polymer units are connected with phenyl ether linkages. The phenyl ether linkages are more resistant to chemical degradation compared with the ester linkages. Additionally, the ester linkages are only located at the end of the vinyl ester molecule which decreases the number of ester linkages in the network. If the vinyl ester is terminated with a methacrylate group, the pendant methyl group provides steric hinderance (or shielding) around the ester linkage thus lower the amount of chemical attack to the ester linkage [1].
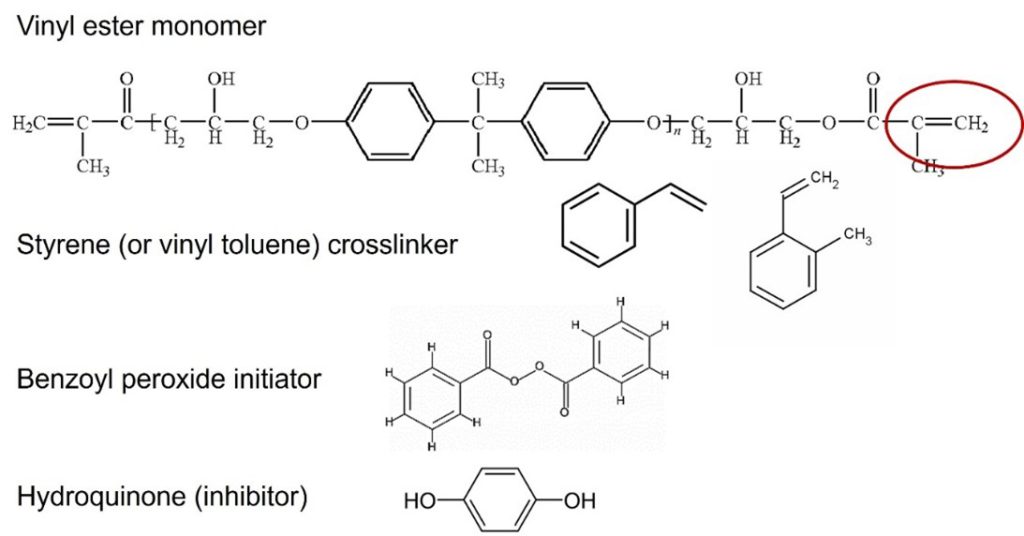
A typical vinyl ester formulation is shown below.
Note that the major components in a vinyl ester formulation are very similar to components used in an unsaturated polyester compound. The liquid styrene containing resins are typically compounded with additives such as catalysts, UV stabilizers, flame retardants, thixotropic agents, fillers, fiber reinforcements, pigments, wetting agents, release agents, and flexibilizers depending on the application and molding process.
Ineos Composites (formerly Ashland Chemical) is a supplier of vinyl ester resins under the tradename of Derakane. The Derakane and Derakane MomentumTM 411 resins are standard vinyl ester resins based on bisphenol-A epoxy resin. Derakane MomentumTM 441-400 resins are low styrene monomer bisphenol A epoxy vinyl ester resins. Derakane MomentumTM 470 series are epoxy novolac-based vinyl ester resins. A very good reference describing the chemistry and applications of the Derakane vinyl esters may be found in [2]. (Note: click on the link to open the pdf file).
References
- Dodiuk, H editor, (2022) Handbook of Thermoset Plastics, Fourth Edition, Oxford, United Kingdom, ISBN 978-0-12-821632-3
- Ineos Derakane Fabrication Guide

Leave a Reply