 This post is the first in a series that will cover the chemistry and curing of thermosets or thermosetting polymers. This post will provide an introduction to the types and applications of thermosets. The image on the left shows a filament wound container. The individual fibers are impregnated with a thermosetting resin (typically epoxy) and then are wound around a mandrel to provide the shape and then cured to achieve the desired final end-use properties.
This post is the first in a series that will cover the chemistry and curing of thermosets or thermosetting polymers. This post will provide an introduction to the types and applications of thermosets. The image on the left shows a filament wound container. The individual fibers are impregnated with a thermosetting resin (typically epoxy) and then are wound around a mandrel to provide the shape and then cured to achieve the desired final end-use properties.
The most common thermoset types (or chemistries) are:
- Epoxy
- Unsaturated polyester
- Vinyl esters
- Phenolics
- Polyurethanes
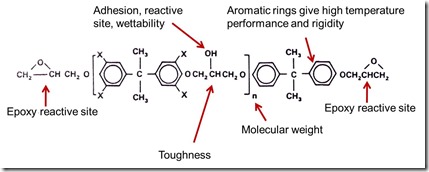
The common feature of thermosets is the molecules contain a chemical moiety that is capable of undergoing a chemical reaction to form a specific type of link called a crosslink. Depending on how many of these functional groups are on a monomer, the chemistry can be tailored to achieve specific final fully cured physical properties. Epoxy is a very common thermoset used in a variety of applications from simple, low-cost adhesives to high engineered electrically conductive adhesives, to structural composites with very high strength. The epoxy molecule is shown in the following figure:
In the epoxy monomer, the epoxy group (or oxirane) is a strained 3-carbon ring that readily undergoes ring opening reactions with a large number of curing agents (or hardeners). Note that there is an epoxy reactive site on each end of the molecule. Other features of the epoxy monomer are also shown in the above figure. The hydroxyl group can aid in adhesion, wetting of surfaces during application/curing, and also serve as an additional reactive site (nucleophile). We will cover the curing chemistry and nucleophiles in an upcoming post. A common epoxy is called diglycidyl ether of bishpenol A (DGEBA) and is produced my several suppliers in various molecular weights. In the figure above, the “n” represents the number of repeat units. A typical epoxy monomer is EPON 828 with an average n=0.1 (essentially no advancement) and has a molecular weight of 380. For n=2 (an example is EPON 1001F) there would be two repeat units and the average molecular weight would be 900. The EPONTM epoxy resins are available from Momentive Performance Materials.
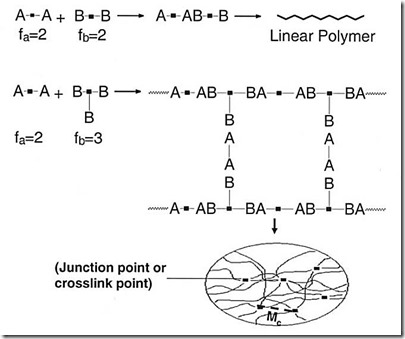
During polymerization of the epoxy group, the choice of the hardener is critical. Typical hardeners have functionality equal to greater than 3. A functionality of 3 is required to get a crosslinked network. In the figure below, one readily observes that if both the epoxy and the hardener are difunctional, then a linear polymer is formed. The use of a f=3 hardener leads to crosslinking and the typical thermoset network.
In typical epoxy crosslinking polymer systems, the schematic above applies in that there needs to be a chemical reaction to link polymer chains together. In future posts we will explore how to tailor the properties by using a “molecular architect” approach.

Don’t forget that there are also thermoset rubbers (e.g. EPR, EPDM) these are critical components to the wire and cable industry.
How to prepare epoxy composites from oil? Which hardner is used to prepare epoxy composites?