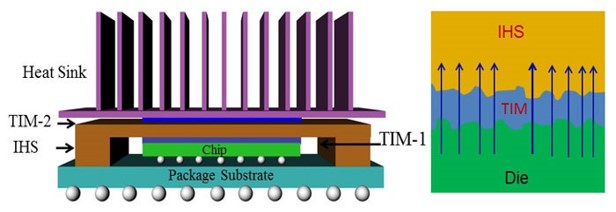
 The two previous posts in this series provided an introduction to thermal interface materials (TIM) and a description of why TIM’s are used to facilitate heat transfer across tow surfaces. This post will cover two types of thermal interface materials:
The two previous posts in this series provided an introduction to thermal interface materials (TIM) and a description of why TIM’s are used to facilitate heat transfer across tow surfaces. This post will cover two types of thermal interface materials:
- Thermally conductive and electrically conductive
- Thermally conductive and electrically insulating
There are many types of materials that are classified as #1 above, notably many silver-filled die attach adhesives are electrically and thermally conductive. The use of silver flakes facilitates both electrical and thermal conductivity due to the plate-like geometry of silver flakes. Aluminum is also used to formulate electrically and thermally conductive adhesives. The challenge in TIM applications is the requirement for electrically insulating and thermally conducting materials.
Polymers are very good insulators and the thermal conductivity (k) of most unfilled polymers is about 0.2 W/mK. In order to increase the thermal conductivity while maintaining electrical insulation, non-conductive fillers must be employed. Some common non-conductive fillers are [1]:
- silica, k = 1.2 W/m•K, spherical
- alumina, k = 36 W/m•K , polygon
- boron nitride, k = 300 W/m•K, platelet
- diamond, k = 2000 W/m•K, polygon
Spherical silica is one of the most common fillers used in a variety of thermosetting compositions such as mold compounds, underfills, and non-electrically conductive die attach adhesives. Silica has a relatively low value thermal conductivity (k = 1.2 W/mK) and while it is about 6X greater than epoxy resin, even at high filler loadings, the thermal conductivity is not increased significantly. In a nice paper on thermally conductive filler in a capillary underfill, Lee and Yu [1] presented data on various types of thermally insulating fillers listed above.
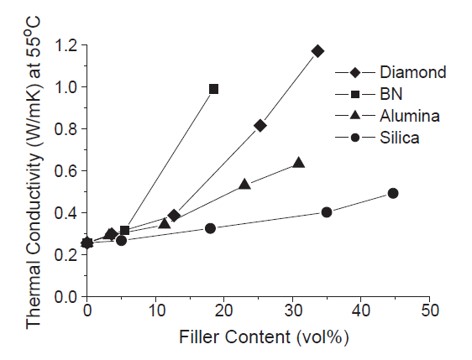
Adding thermally conductive fillers can increase the composite thermal conductivity as seen in Figure 1.
Figure 1. Thermal conductivity versus filler content for an epoxy anhydride resins system used for capillary underfills (from [1])
The composite thermal conductivity is influenced by the filler loading, filler k, and the filler aspect ratio. In the list above, the values for k and a description of the aspect ratio is provided. In Figure 1, the thermal conductivity is plotted as a function of filler content in volume percent. The polymer matrix was an epoxy anhydride resin system with a k =0.23 W/mK [1]. As shown in Figure 1, boron nitride (BN) was the most effective filler for increasing thermal conductivity at low loading levels. Note that diamond has a k = 2,000 W/m•K and BN has a k =300 W/m•K , but the boron nitride is more efficient at increasing the composite thermal conductivity. This is observed in Figure 1 at 15 volume percent filler loading where the BN thermal conductivity is approximately twice that of the diamond containing composite. In this case, filler aspect ratio or geometry plays an important role in the development of thermal conductivity. The morphology of BN is plate like [2]. In the work of Lee and Yu [1], they used scanning electron microscopy to investigate the filler morphology used in the epoxy anhydride underfill formulation.
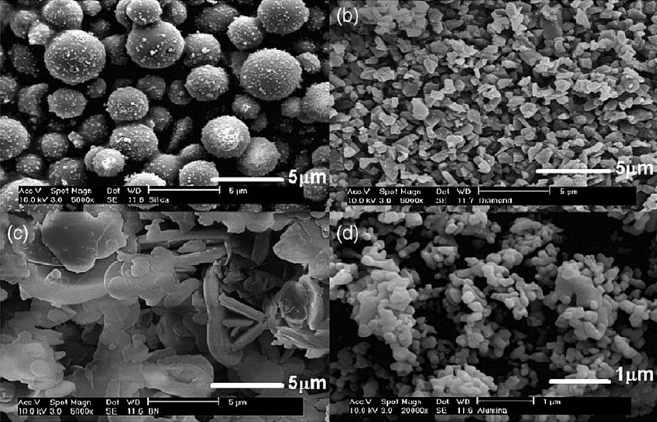
Scanning electron microscopy (SEM) was used to characterize the filler morphology and SEM micrographs are shown in Figure 2 for the four types of non-conductive fillers listed above.
Figure 2. SEM micrographs of fillers: (a) silica, (b) diamond, (c) BN, all at 5,000x, (d) alumina at 20,000x. [Ref 1]
In the upper left, the spherical nature of the silica filler is obvious. In the lower left, the plate like morphology of the BN fillers is shown. For the diamond and the alumina (upper and lower images on the right side in Figure 2), the particle morphology is termed polygon (that is, not spherical but not plate like). Due to the efficacy of the BN filler interactions, the thermal conductivity increased nearly 5-fold at 15 volume percent loading. At this loading, as seen in Figure 2, the thermal conductivity of the BN filled composite is approximately twice that of the diamond filled composite even though the thermal conductivity of diamond is nearly an order of magnitude larger boron nitride. The data clearly demonstrates the role of the filler aspect ratio and geometry on the thermal conductivity of the filled composite.
References
- Woong Sun Lee and Jin Yu, Diamond and Related Materials 14, 1647 (2005).
- Sato, H Horibe, T. Shirai, Y. Hotta, H. Nakano, H. Nagai, K Nitsuishi, and K. Watan, J. Mater. Chem. 20, 2749 (2010).

Leave a Reply