Guest Post by Dr. Robert (Bob) Humphreys
In Post 11 in this series, we gave a short overview of plastic-to-syngas, one of several technologies for conversion of waste plastic to useful products. Syngas is a valuable product because it can be burned as a fuel or can be converted into liquid fuels (diesel, gasoline) or methanol that, in turn, can be converted to a broad range of basic and commodity chemicals including monomers that are the building blocks of plastics (one approach to “the circular economy”). However, as useful as syngas can be, transformation into liquid fuels and chemicals requires that syngas be compressed to high pressure, particularly if the gas is to be shipped to an alternative site for processing, and compression requires considerable energy (= additional cost for energy and equipment). Conversions discussed below produce products that can be used directly or are liquids can be shipped easily.
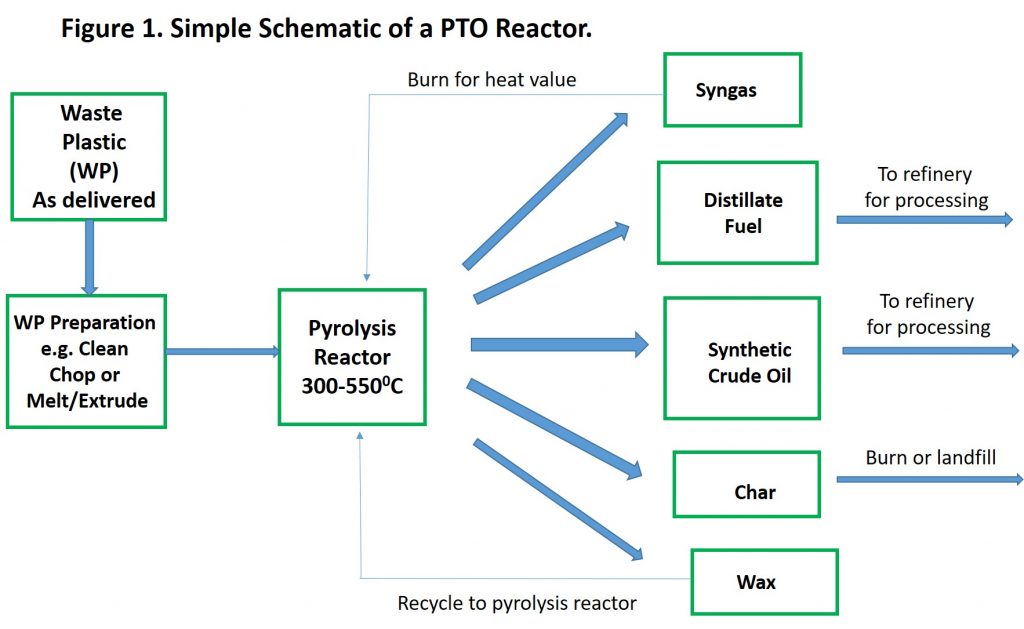
Plastics-to-Oil (PTO), an Alternative to Syngas
High volume waste plastics such as high- and low-density polyethylene (HDPE and LDPE, respectively), polypropylene (PP), and polystyrene (PS) have a high hydrogen to carbon ratio that makes them excellent raw materials for what is known as plastic-to-oil (PTO) conversion. PS content can add to the aromatic content of the liquid products from the pyrolysis, which is desirable. PTO is a high temperature process (typically 300-5500C, see ref. 1), but lower than for syngas production (>5500C, can be up to 10000C). PTO is conducted in the absence of oxygen and with or without added steam (see ref. 2 for some examples of conditions) and catalysts are used in some processes. A range of products can be produced, depending on the conditions of the process, but the product range from a generic process would include syngas, synthetic crude oil, distillate fuels, and char. Reported conversion to liquid products (distillate, synthetic crude) are in the range of 60-80%, with lower amounts of syngas (6-20%), wax (10%), and char (5-20%). Clearly, there is potential for efficient conversion.
Syngas from the process can be captured and used as a source of heat for the pyrolysis reactor (i.e. burned) or flared. The fate of the wax is usually recycle back through the pyrolysis process (i.e. added to the waste plastic stream). Char can include carbon from the pyrolysis and any solid materials that were not removed from the waste plastic that cannot be pyrolized (e.g. glass shards, metal fragments). The carbon in the char has burn value (similar to coke from a refinery) but can have other fates (e.g. landfilling). If the char is burned for energy, then PTO can be viewed as an approach to high-efficiency recycling of waste plastic, although as noted in ref. 3, local regulations can make classification of pyrolysis as recycling uncertain. Liquid products from the pyrolysis can be of high quality (e.g. low sulfur content) and can be added to processing streams in a petroleum refinery to provide a revenue stream to the pyrolysis company, thereby converting the pyrolysis products to higher value fuels and chemicals, including monomers for plastics.
Preferred Waste Plastics for PTO:
One of the advantages claimed for PTO is ability to deal effectively with lower quality waste plastic that is not acceptable for customers of plastic recyclers for reuse in consumer product packaging. This means that PTO may be an effective way to use the massive amount of waste plastic that is unacceptable for recycling. Since only about 9% of waste plastics are recycled in the USA out of over 30 million tons, there is little doubt that there is ample potential feedstock once effective separation is achieved (note: global waste plastics estimate is 130 million metric tons, see 2015 ref. 3).
Of course, issues for PTO exist. For example, PTO facilities prefer not to have PET in the waste plastic (plastic recycle #1) since the high level of oxygen present in PET can reduce the quality and yield of liquid products. This may not be a large issue since PET is the highest value plastic for recycle/reuse. PTO facilities also prefer to avoid nylons because of their high nitrogen content and thermoset resins. PVC (plastic recycle #3) is also undesirable because PVC chlorine content produces hydrochloric acid when pyrolized, which necessitates scrubbing to remove the acid and may affect the other desirable products. Apparently some PTO processes can effectively deal with PVC but avoiding it is preferred.
HDPE presents an interesting problem. HDPE (plastic recycle #2) is the other recycled plastic that significant value, with about 30% being recycled (versus 28% for PET). Reports of shortages of high quality PET and HDPE suggest that these numbers may grow. Since HDPE is a preferred feedstock for PTO facilities, there may be competition for this material in the future. Nevertheless, considering the overall low level of plastic recycle/reuse, lack of markets for recycled LDPE and PS, relatively little PP recycle/reuse compared to PET and HDPE, and the vast amount of waste plastic film (mostly LDPE, HDPE, PP) that is landfilled or incinerated, there is a huge potential feedstock for PTO that is currently untapped.
Depolymerization: Another Way to Recycle
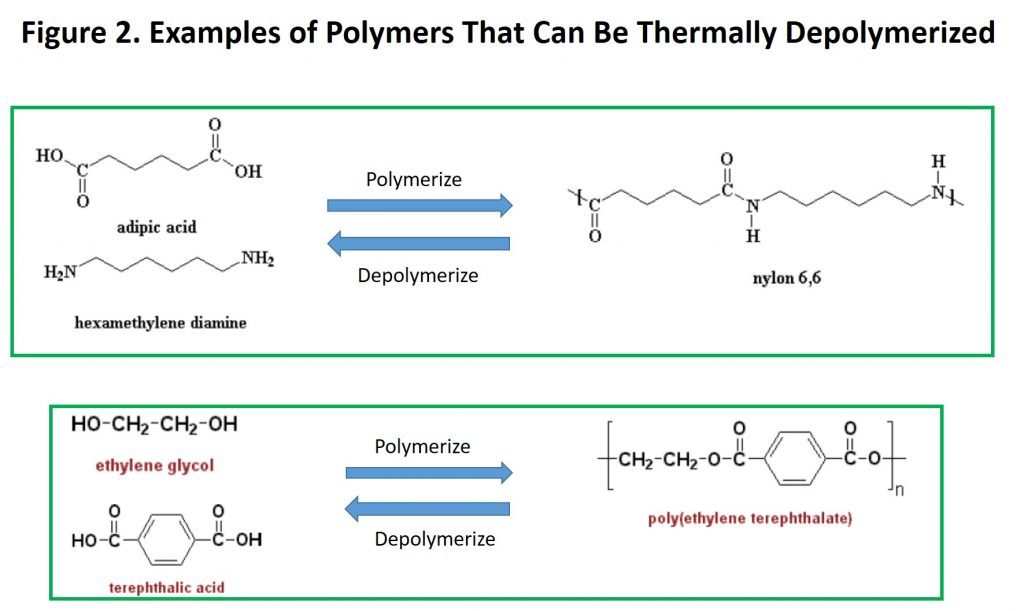
Up to this point, the thermal methods we have discussed are highly destructive, converting waste plastic into either gases like hydrogen and carbon monoxide (the major syngas components) or liquid products that resemble fractions from crude oil refining. There is yet another thermal process that converts waste plastic back into the monomers from which it was produced, a process called thermal depolymerization. For example, both nylon-6,6 and polyethylene terephthalate (PET) can be converted into their respective monomers, as shown in Figure 2. Polyurethane foam also can be thermally depolymerized. Ref. 4 provides a list of companies that have depolymerization technology.
The reader may have noticed that preferred waste plastics for thermal depolymerization also are plastics that are to be avoided for PTO, suggesting that as recycling continues to develop, recycle/reuse, pyrolysis to syngas, PTO, and thermal depolymerization all may be part of an effective system.
In the next and final post in this series, we will give a short summary of the state of waste plastic recycling and give an indication of how the many parts might fit together into a coherent whole in the future.
References


Very interesting technologies and most appropriate to curb environmental pollution. Studies should be enhanced to come up with sustainable solutions to plastic wastes since these are on the market and on high demand
This article sheds light on the urgent need to address the issue of plastic waste and its impact on our environment. It’s high time we take action and find sustainable solutions that can help us make a positive impact on our planet