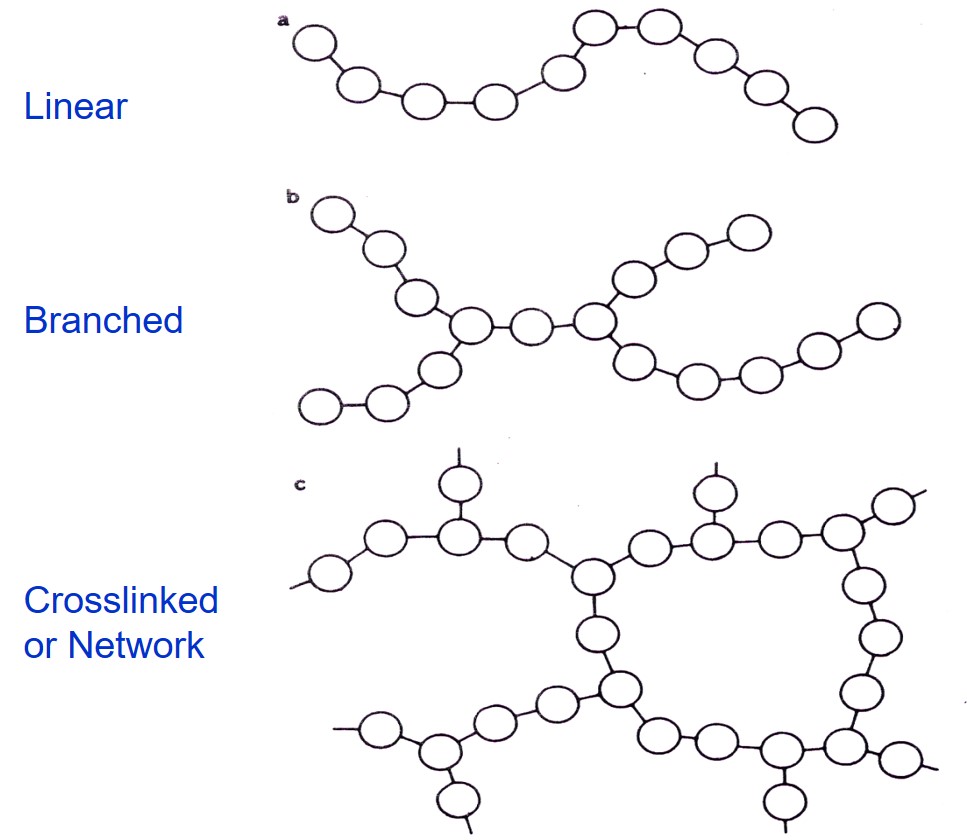
 Polymers are a key enabler in electronic packaging. The logical question is: what types of polymers are well-suited for electronics? There are two basic classes of polymers; thermoplastics and thermosets. As shown in the schematic on the left, polymers (poly meaning many, and mers meaning units) are long chains of repeating chemical units. Polymers can be linear (polystyrene, polypropylene), branched (polyethylene) or crosslinked (epoxy).
Polymers are a key enabler in electronic packaging. The logical question is: what types of polymers are well-suited for electronics? There are two basic classes of polymers; thermoplastics and thermosets. As shown in the schematic on the left, polymers (poly meaning many, and mers meaning units) are long chains of repeating chemical units. Polymers can be linear (polystyrene, polypropylene), branched (polyethylene) or crosslinked (epoxy).
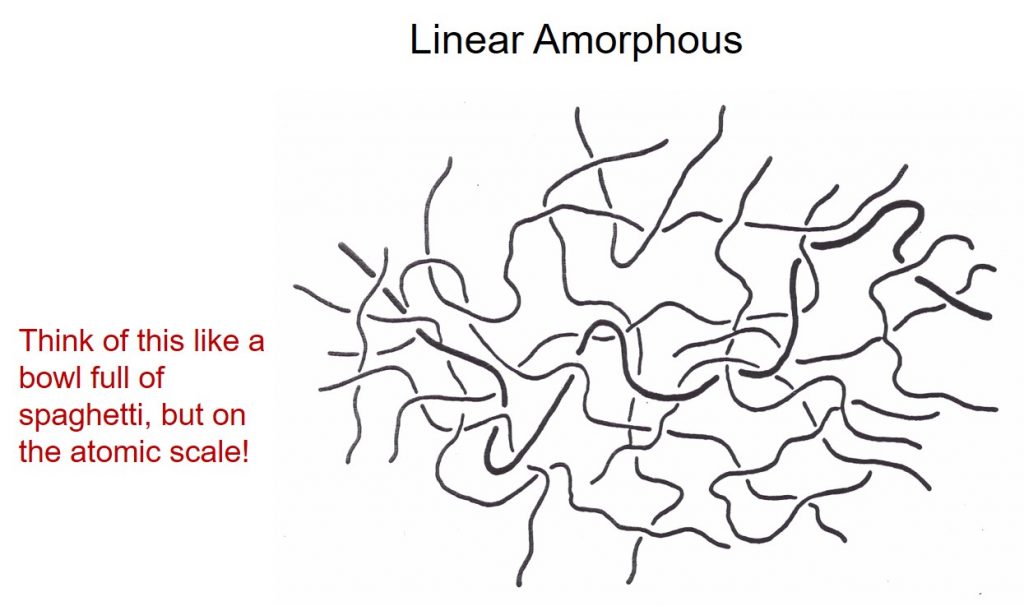
Thermoplastics are very long polymer chains with a high molecular weight and can be either crystalline or amorphous. The high molecular weights of polymers impart their useful properties such as good mechanical properties and have the ability to be molded into various types of parts (injection molded, extruded, etc). Thermoplastics can be amorphous (no crystallinity) and can be considered to be “like a bowl full of spaghetti” with the long chains entangled with each other. The late Professor Garth Wilkes from Virginia Polytechnic Institute used to refer to molten polymers (like linear amorphous) and a bowl full of snakes, since the polymer chains are undergoing random Brownian motion and slithering past one another. A schematic of a linear amorphous thermoplastic is shown in the following figure.
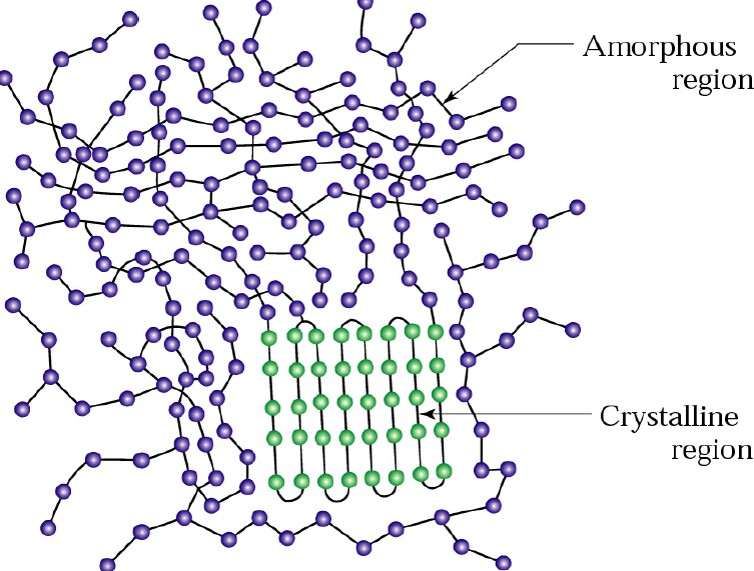
Polystyrene is a good example of a linear amorphous thermoplastic. Polystyrene has mid-range mechanical properties with a glass transition temperature (Tg) of 100°C and since it is amorphous is clear (some of the typical hard, clear disposable drinking cups are PS). Linear amorphous polymers only have a Tg (since there is no crystalline phases to melt) and above Tg they will soften and flow. Semi-crystalline thermoplastics have both an amorphous region and a crystalline region. The amount of the crystalline phase typically determines the physical and mechanical properties. Semi-crystalline polymers have a melting point (i.e. the temperature where the crystalline domains melt) and a Tg corresponding to the amorphous region. The following schematic shows an idealized morphology for a semi-crystalline polymer.
Semi-crystalline polymers have a very wide range of melting points that are typically under 200°C (except for the high performance engineering (and expensive) thermoplastics). Semi-crystalline polymers have several attractive physical properties (good modulus, toughness, low moisture absorption) but are very difficult to process (remember the long chains). The main drawbacks to using thermoplastic polymers in electronic packaging are twofold:
- As the molecular weight increases, the viscosity increases very rapidly requiring processing at temperatures above the melting point
- In use, if the Tg or Tm is exceeded, the dimensional stability (i.e. ability to flow) limits the use in electronic packaging, since most of the electronic packages experience multiple solder reflows where the temperature is likely to be in the range of 220°C – 260°C, well above the Tm or Tg of most thermoplastics.
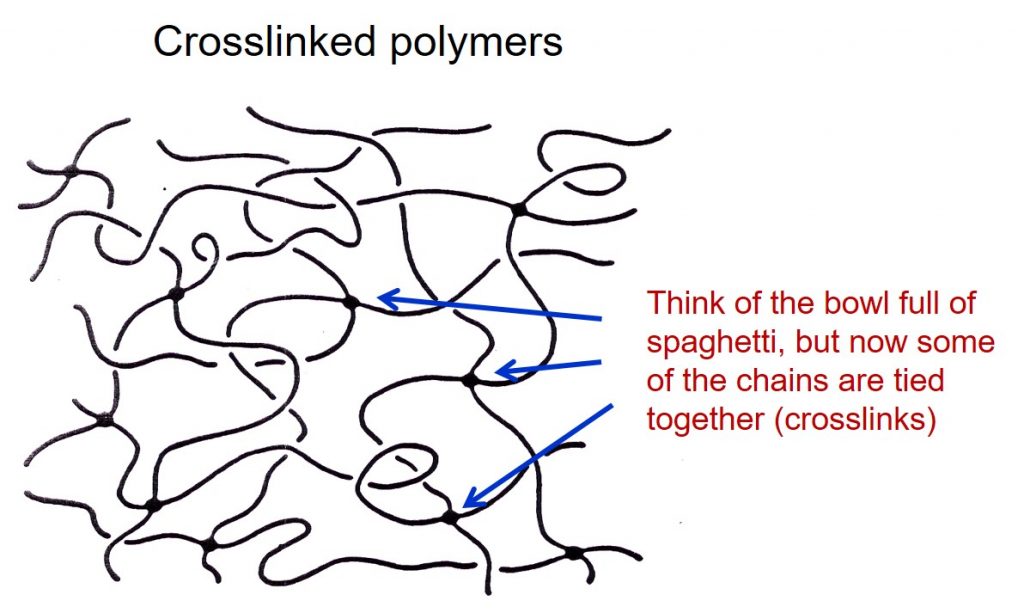
Thermosets are a class of polymers that start out as small molecules (monomers and oligomers) and during a chemical reaction polymerize into a network structure. In the final fully cured state, the crosslinks join the chains together providing both good mechanical properties, but thermosets will not flow (and be dimensionally stable) above the Tg (thermosets only have Tg’s). A schematic of a crosslinked network is shown in the following schematic.
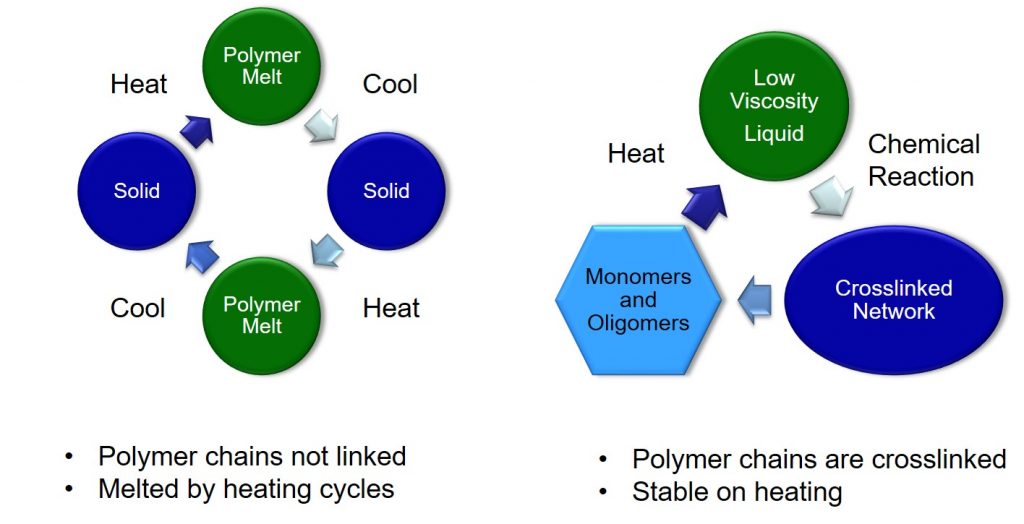
Crosslinks prevent the chains from sliding past one another resulting in a stable material that will not flow above the glass transition temperature. Subsequent posts will cover more on the crosslinking reactions and network formation, which is a critical difference between thermosets and thermoplastics. The following schematic compares the processing of thermoplastics (left) with thermosets (right).
In the figure on the left, a thermoplastic polymer may be heated above the glass transition or the melting temperature resulting in a highly viscous liquid that can be formed into useful shapes using heat and pressure. Upon cooling, the polymer will either crystallize or turn into a glassy polymer (solid). Subsequent heating will repeat the process. This is why many linear thermoplastics (such as polyethylene terephthalate (PET) used in water bottles) can be recycled.
Thermosets (schematic on the right) start out as small molecules (monomers and oligomers) and during heating turn into a very low viscosity liquid. The heat actives a series of chemical reactions that cause the growth of polymer chains and then ultimately forms a fully cured, crosslinked network. On subsequent heating (for example during 240°C reflow to attach solder balls), the crosslinked network will not flow or loose it shape. Once the network is formed, the chemical structure does not revert back to monomers or oligomers unless the temperature exceeds the degradation temperature.

Leave a Reply