Guest Post by Dr. Robert Humphreys
In previous posts, Dr. Gotro discussed properties and commercial applications of poly (L-lactic acid), or PLLA. PLLA of high molecular weight and very high L-lactic acid content (i.e. very low D-lactic acid) is required for most commercial applications. This post will focus on how the PLLA polymer is made.
PLLA is a linear polyester, which is a polymer formed by reaction between difunctional monomers with alcohol and carboxylic acid functional groups. Ester formation between a carboxylic acid and an alcohol is a “condensation reaction”, producing water as a co-product which is usually removed by distillation to drive the reaction to completion. A strong acid catalyst and elevated temperature are usually required for an esterification reaction to occur at a practical rate. Condensation polymers such as polyester are formed step-wise, one ester bond at a time. Consequently, high molecular weight is achieved only towards the end of the reaction when most of the monomer has been consumed and further reaction involves predominantly polymer molecule end groups. Impurities that react with alcohol or carboxylic acid groups can limit the fraction of high molecular weight polymer achievable by blocking one or both reactive ends of growing polymer chains.
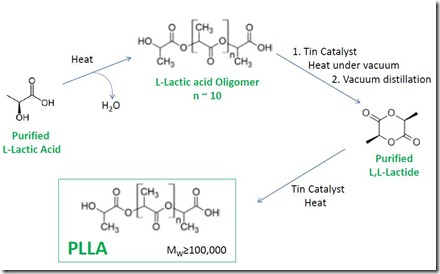
Lactic acid is a difunctional monomer with both carboxylic acid and alcohol functions and is capable of self-condensation to give polyester. Nevertheless, because a high yield of high molecular weight polymer is required to meet economic and performance requirements, the preferred commercial process involves prior formation of “lactide”, the cyclic diester formed from two molecules of lactic acid with loss of two molecules of water. The need for PLLA with very high L-lactic acid content means that high-purity L,L-lactide must be used, which in turn requires high purity L-lactic acid. L,L-lactide is produced by heating purified L-lactic acid to form low molecular weight “pre-polymer” (oligomer) while driving off the water. The pre-polymer is then “cracked” in the presence of a tin catalyst to give L,L-lactide, which is removed by distillation. Efficient vacuum distillation produces L,L-lactide of sufficient purity for PLLA production. Purified L,L-lactide can then be polymerized in the presence of a tin catalyst to high molecular weight PLLA that is suitable for commercial applications described in previous posts. Catalyst type and polymerization conditions are controlled carefully to minimize isomerization at the chiral carbon, since even small amounts of the D-lactate stereoisomer in the PLLA have a negative effect on commercially important polymer properties. Trace hydroxyl-containing impurities can be involved in polymerization initiation and impact the maximum molecular weight attainable in the polymerization. A simplified version of the overall process is shown in the following figure.
It should be apparent from the information in this and the previous post that successful commercialization of PLLA has required development of extensive technical know-how, including:
– An efficient fermentation for L-lactic acid, including proprietary microorganisms, production, isolation, and purification technologies, all on a huge scale
– High-yield production and purification of L,L-lactide
– Polymerization of L,L-lactide to high molecular weight poly(L-lactide) with very high L-content
An efficient sugar supply chain to feed the fermentation and extensive polymer applications know-how also support a business based on PLLA. In many ways, PLLA provides a model for successful commercialization of a renewable, bio-based material that is new to the market place.
References:
1. Poly(lactic acid). Synthesis, Structures, Properties, Processing, and Application. Rafael Auras, Loong-Tak Lim, Susan E.M. Selke, and Hideto Tsuji, Editors. John Wiley and Sons, Hoboken, NJ. 2010.
2. Patents: US5338822, US5521278, US5274073.
I would like to thank Dr. Humphreys for his guest contributions on the chemistry of biopolymers and some of the key issues surrounding adoption of biopolymers and bioplastics from a chemistry perspective.
In the next few blog posts, we will transition to discussions about innovation and product development, the key to getting our economy moving again.


The recycling of PLA filaments is still a great problem. PLA needs to be recycled at high temperatures under controlled industrial composting conditions to break down properly. I wonder whether it is biodegradable or just degradable.
PLA filament is the most popular choice in the 3D printing community. Its affordability, ease of use, and the availability of various modified PLA options that offer new features make it a favorite among users.