The previous posts in this series covered various types of bismaleimide resins and formulations. The next three posts will discuss cyanate ester resins and formulated products.
Dicyanate resins, alternatively known as cyanate ester resins, describes both a family of monomers and oligomers with reactive cyanate end groups on an aromatic ring and the resulting cured resin networks (see review by Kessler [1]). Bisphenol A dicyanates were commercialized in the late 1970s and were initially used in high performance circuit boards. During initial testing, exposure to high relative humidity caused moisture stability problems. Moisture resistance was improved by blending bisphenol A dicyanates with epoxy resins to reduce the amount of ester linkages in the resulting copolymer. During the 1980s and 1990s new dicyanate ester monomers were developed [2], including a bicyclopentadiene containing dicyanate (Dow 71787.02) [3]. The bicyclopentadiene modified cyanate exhibits excellent electrical and mechanical properties and enhanced processability.
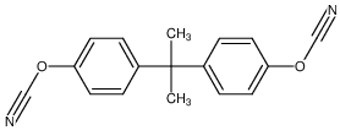
The most common cyanate ester is the bisphenol A dicyanate as shown in Figure 1.
Figure 1. Bisphenol A dicyanate
The characteristic of all dicyanates is the presence of the triple bond and in the case of Bis A dicyanate, there are two cyanate end groups. The cyanate end groups undergo a cyclotrimerization reaction forming high Tg, rigid thermosets. Bisphenol A dicyanates have high Tg‘s but were brittle and exhibited low fracture toughness. Several approaches were developed to toughen cyanate esters including the use of core/shell rubbers [4] and thermoplastic toughening agents such as polyethersulfone, polysulfone, and polyetherimides [4, 5].
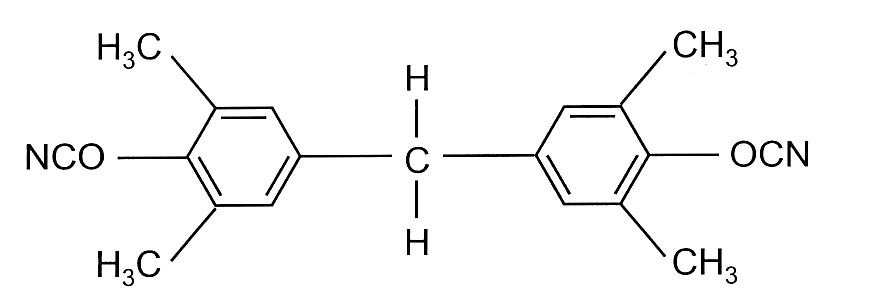
Tetramethyl bisphenol F dicyanate is a liquid version used where low viscosity is required and is shown in Figure 2.
Figure 2. Tetramethyl bisphenol F Dicyanate (liquid dicyanate)
An additional benefit of the tetramethyl bis F dicyanate is lower moisture absorption from the steric hinderance from the methyl groups near the cyanate group. Liquid cyanate ester monomers (bisphenol F dicyanates) have low viscosity at 25°C and exhibit good compatibility with other cyanate ester resins, epoxy resins, and bismaleimide resins [6]. Blending liquid cyanate ester resins with liquid epoxy resins provided a pathway to control the rheological properties without sacrificing the final cured performance. Liquid cyanate esters enabled the development of underfill resins for chip packaging applications, where low viscosity is required during processing. After curing, cyanate ester resins provide high Tg, low coefficient of thermal expansion, high toughness, and low moisture absorption required for underfill applications.
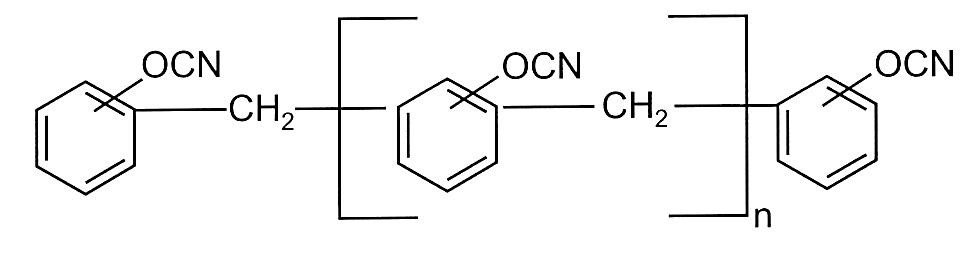
There are several other more exotic types of cyanate ester monomers including multi aromatic ring dicyanates as shown in Figure 3.
Figure 3. Phenol novolac cyanate ester
The phenol novolac cyanate ester shown in Figure 3 was commercialized by AlliedSignal under the tradename PT resins. PT resin contained a blend of multifunctional phenolic cyanate/phenolic triazine copolymer and an epoxy resin [7]. The cured resin blend has a high Tg and flexural strength along with low moisture absorption properties.
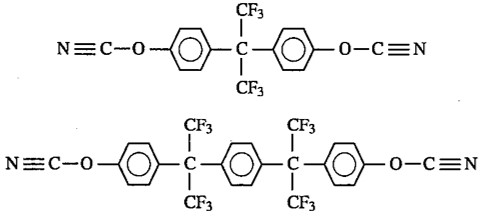
Fluorine containing cyanate esters were used in electronic packaging applications [8] leading to crosslinked networks with very good electrical properties, specifically low Dk. Figure 4 shows the chemical structure of two types of fluorine containing cyanate esters.
Figure 4. Fluorine containing cyanate esters.
The fluorine containing monomers were very expensive and as such limited the use to only very specific packaging applications. The use of fluorinated cyanate esters for use in electronic packaging applications will be presented in the final post in this series.
The predominant curing pathway for cyanate ester is via cyclotrimerization using metal coordination catalysts. Three types of metal coordination catalysts are used:
- metal naphthenates
- metal acetylacetonates (AcAc)
- metal octoates
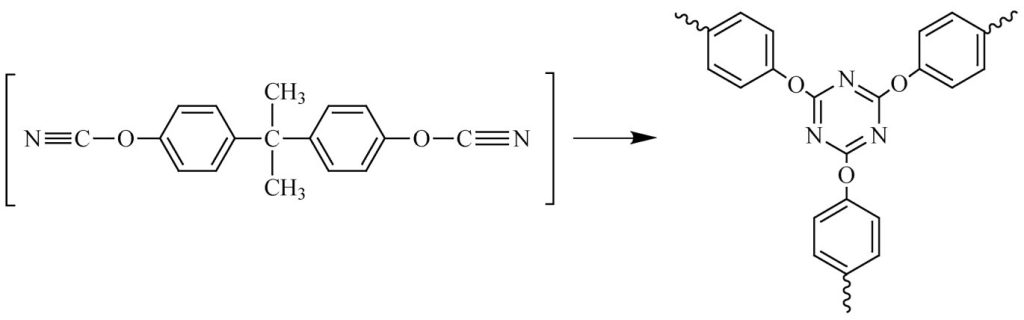
Of these, copper acetylacetonate abbreviated Cu(AcAc), cobalt (II) acetylacetonate, Co(AcAc) and Mn octoate are the most popular coordination catalysts. Nonylphenol is employed as a co-catalyst. The cyclotrimerization reaction of bisphenol A dicyanate to form the triazine network structure is shown in Figure 5 [2].
Figure 5. Cyclotrimerization reaction forming a highly rigid cyanate ester thermoset.
In order to achieve very high Tg, dicyanates are cured without addition of epoxy modifiers leading to a very tight network via cyclotrimerization. Due to the highly aromatic ring structure, as shown on the right in Figure 3, homopolymerization leads to thermosets with high Tg, but brittle networks. Additionally, the ring structure leads to free volume resulting in a thermoset network with high moisture absorption. On the positive side, the low polarity of the cyclotrimerized network leads to a thermoset with low dielectric constant (Dk) and low dissipation (or loss factor) Df.
The advantage of using cyanate ester monomers is the ability to formulate with a wide range of epoxy resins. The extensive epoxy resin formulation toolbox enables a large number of options for the formulator to achieve the desired physical properties such at Tg, modulus, moisture absorption, and fracture toughness. Typical resins in electronic applications are combinations of specific epoxy monomers and oligomers along with cyanate esters.
The very good electrical properties were the driving force to develop formulations for electrical applications. Mitsubishi Gas Chemical pioneered the development of circuit substrates by blending bisphenol A dicyanates with epoxies and a small amount of bismaleimide. The resulting bismaleimide/triazine/epoxy (BT epoxy) laminates are the industry standard for low dielectric, high Tg chip packaging substrates and will be discussed in the next post.
References
- R. Kessler, Cyanate ester resins in Wiley Encyclopedia of Composites, (Nicolais L, editor), John Wiley & Sons, Inc. (2012).
- A. Shimp, in I. Hamerton, ed., Chemistry and Technology of Cyanate Ester Resins, 282, Chapman and Hall, Glasgow, U.K., 1994, Chapt. 10.
- Plastics Technology, 8 (1988).
- C. Yang, D. Pickleman, and E. Woo, 35th Int. SAMPE Symp. 1131 (1990).
- Shimp and W. Craig, 34th Int. SAMPE Symp. 222 (1989).
- US Patent 4,110,364, Morio Gaku et. al.
- US Patent 2002/0010286 A1, 2002 AlliedSignal PT resin
- US Patent 5,527,838, Gotro et. al.

Leave a Reply