Guest Post by Dr. Robert Humphreys
In previous posts, we described how biomass is converted to bio-refinery friendly forms of feedstock and how these feedstock forms are converted into basic chemicals. We emphasized two basic chemical products, p-xylene (pX) and 5-hydroxymethylfurfural (HMF) since they can be used to make monomers for production of bio-plastics that are potential replacements for petroleum-derived plastics in beverage bottles. We are now on the final leg of our journey from wood chips, switch grass, corn cobs, and yard clippings to renewable plastic beverage bottles: how are biomass-derived pX and HMF converted into renewable monomers and, subsequently, renewable plastics?
From bio-pX to bio-PTA
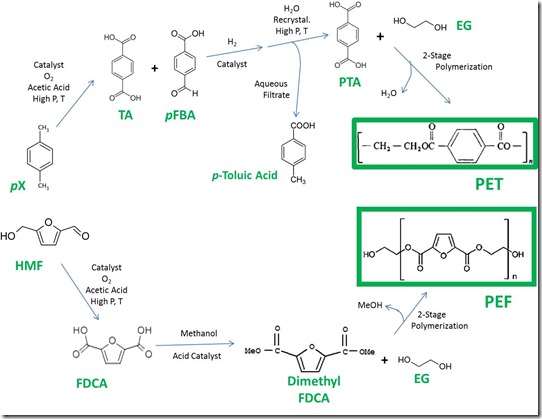
Bio-pX is an example of a renewable equivalent to a chemical that is traditionally produced from petroleum. Technology for large-scale separation and purification of petroleum-derived pX is well developed and can be applied to bio-pX. Thus, bio-pX can potentially be “dropped into” the process for conversion of pX to terephthalic acid (TA) and purified terephthalic acid (PTA), the form of TA of sufficient purity to manufacture polyethylene terephthalate (PET) for beverage bottles. Briefly, pX is oxidized to TA with oxygen and a catalyst in acetic acid. The current process is an optimized version of technology developed in the 1950’s. This catalytic oxidation produces a small amount of an impurity (p-formylbenzoic acid, pFBA) that must be removed. Catalytic hydrogenation converts pFBA to p-toluic acid, which stays in water during purification of TA by recrystallization. The recrystallization process is conducted in water at high temperature and pressure because of the low solubility of TA in water. Recrystallization is critical to transforming TA into PTA for bottle-grade PET. Substitution of bio-pX for petro-pX should give bio-PTA that can be used to make bottle-grade plastic. Of course, large-scale production of bio-PTA must await development of a commercial process of comparable scale for bio-pX.
From HMF to FDCA
Oxidation of HMF is required to produce furan-2,5-dicarboxylic acid (FDCA), a monomer that can be used to make bio-plastic for bottles. This conversion is illustrated in the accompanying graphic. The oxidation process is similar to the one used for conversion of pX to TA. Unlike oxidation of bio-pX, there is currently no commercial process for oxidation of HMF to FDCA at a scale required to serve the market for plastic beverage bottles. Nevertheless, there is a substantial body of literature describing catalytic oxidation of HMF to FDCA using metal catalysts and mild conditions. Avantium has achieved pilot-scale production of FDCA.
From Renewable Monomers to Bio-Plastics
Once monomers such as PTA, FDCA and ethylene glycol (EG) are available from renewable sources, the last step in the chain, namely production of bottle-grade plastic, can be taken. We will focus first on PET since the petro-based version of this commercial polymer is used for such everyday applications as clear film, fibers, and, of course, plastic bottles.
PET is a condensation polymer, which means that a small, relatively volatile molecule is generated during the polymerization process. PTA and EG are reacted directly in a melt polymerization using a catalyst, producing water (the small, volatile molecule) which is removed to drive the reaction to completion. The polymerization is conducted in two stages, the first to make a “prepolymer” of low molecular weight and the second to drive the polymerization to high molecular weight. Manufacture of PET is one of the most highly optimized processes in the chemical industry and generates polymer with very low color and high clarity required for beverage bottles. “Drop-in equivalent” bio-PTA and bio-EG can be used to make 100% renewable PET (bio-PET). Obviously, even partial replacement of petro-based PET with bio-PET will require bio-PTA and bio-EG in immense quantities that are not available currently. Toray has made 100% bio-PET using bio-pX supplied by Gevo and bio-EG.
Polyethylene furanoate, or PEF, also is a condensation polymer. Apparently, the preferred process for making PEF involves catalyzed polymerization of the dimethyl ester of FDCA with bio-EG; methanol is removed as the small, volatile molecule. Use of FDCA instead of the diester apparently results in polymer of lower purity. As with PET, the polymerization is carried out in two stages to build the required molecular weight for making plastic bottles. Avantium announced pilot-scale PEF production in early 2011 and has produced PEF bottles.
Processes for PTA and PEF are shown in the graphic above; space limitations have necessitated some simplification. We note that the process for PET is highly optimized in comparison to that for PEF, which has been made only at the pilot scale. Consequently, far more information is available for the PET process, as reflected in the discussion above.
Summary
In this series of four posts, we have tried to present a picture of how raw biomass can be converted to renewable plastics for beverage bottles. Our examples illustrate two very different approaches to development of renewable chemicals: replacement of petroleum-derived chemicals and plastics with “drop-in replacement” renewable versions generated from biomass; and, development of new, renewable chemicals and plastics from biomass that cannot be made readily from petroleum sources. Bio-PET is an example of the former. PEF is an example of the latter, as is polylactic acid (PLA), a polymer that was discussed in some detail in a previous post on this site. It is not our intention to take sides in this battle and, indeed, there may be winners in both camps. We have faith that the marketplace will effectively sort out the winning and losing technologies.


Leave a Reply