This post is part two in a series on high performance thermosets and will cover aromatic bismaleimide curing chemistry.
Bismaleimides are thermosetting polyimides that cure by addition reactions that avoid formation of volatiles. Unlike condensation polyimides (such as DuPont Kapton® or Ube Upilex®) where the polymerization mechanism results in the evolution of volatiles (mainly water) during curing and are typically used as thin films. The bismaleimide resins discussed in this post contain unsaturated double bonds that can be cured via reactions such as:
- Michael addition
- Ene-Alder
- Cycloaddition mechanisms, such as Diels–Alder
- Free-radical
Thermal polymerization without the resultant evolution of volatile by-products enables bismaleimide resins to be used in many applications from electronic materials (such as die attach adhesives), coatings, films, structural adhesives, encapsulants, and high-performance fiber-reinforced composites.
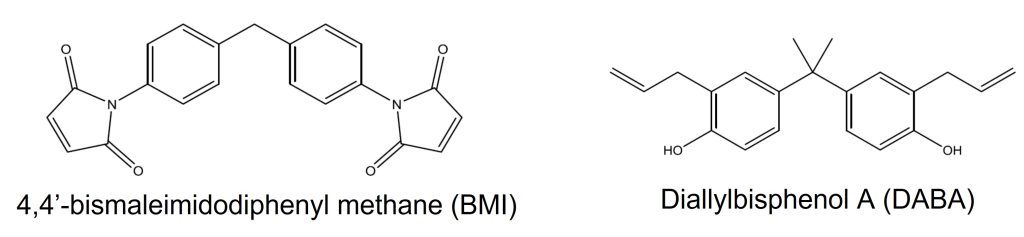
Commercially available resin systems are typically two-component systems. For example, the Matrimid 5292 system from Huntsman Chemical (Matrimid 5292 originally developed by Ciba Geigy) is a blend of BMI and o,o′-diallyl bisphenol A (DABA). The chemical components of the Matrimid 5292 (parts A and B) are shown below.
DABA has low viscosity and is used in place of solvents to enhance the processability and increase the toughness of BMI composites. DBA copolymerizes with BMI causing a decrease in the crosslink density, leading to a tougher network. BMI resins for composites are cured at temperatures in the range of 200–250°C.
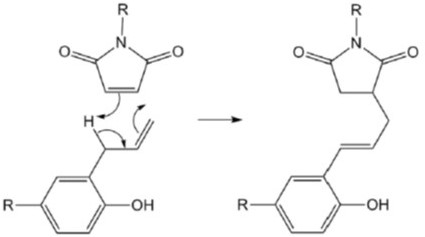
The proposed reaction mechanism for the BMI and DABA is via an ene addition [1, 2]. Figure 1 shows the proposed mechanism from Iredale, Ward, and Hamerton [2].
Figure 1. Schematic of the ene-addition reaction between allyl and maleimide groups (note: half of each molecule is shown) from [2].
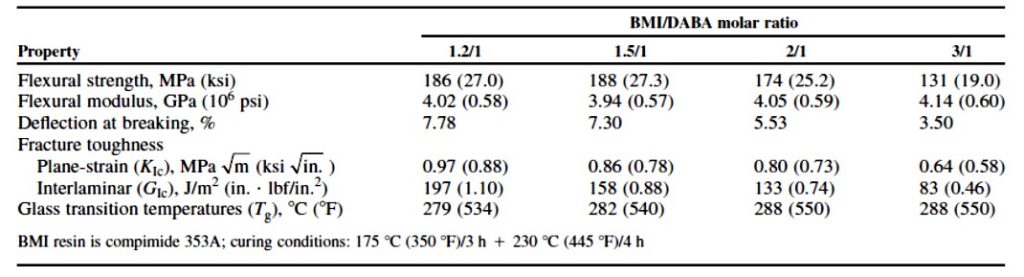
Table 1 shows the properties of binary mixtures of BMI/DABA [1].
TABLE 1
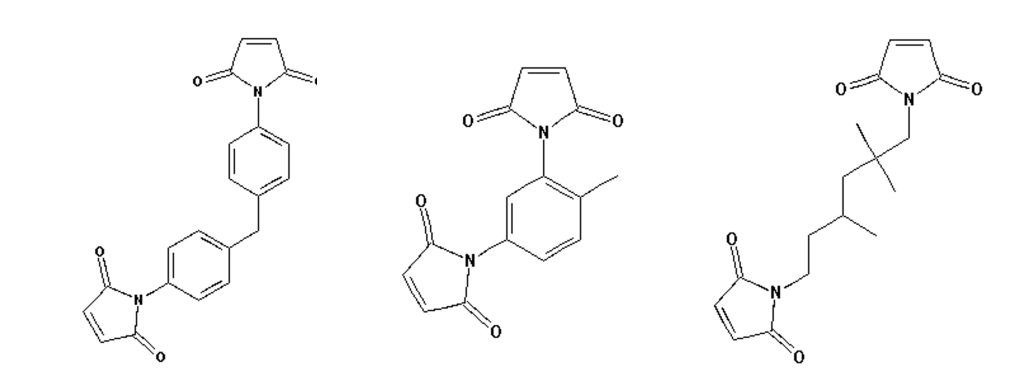
The data in Table 1 used COMPIMIDE® 353 bismaleimide resins which are available from Evonik. The COMPIMIDE® 353 bismaleimide is a mixture of three bismaleimides and the chemical structures are shown in Figure 2.
Figure 2. Chemical structures of COMPIMIDE® 353 bismaleimide [2,3].
The main drawback of highly aromatic BMI resins are low fracture toughness. As shown in Table1, the fracture toughness (both G1C and K1C) is maximized at the 1.2/1 molar ratio of BMI/DABA. As the amount of the BMI component is increased, the fracture toughness decreases. Interestingly, the glass transition temperature (Tg) only increases slightly, and the flexural modulus also only slightly increases.
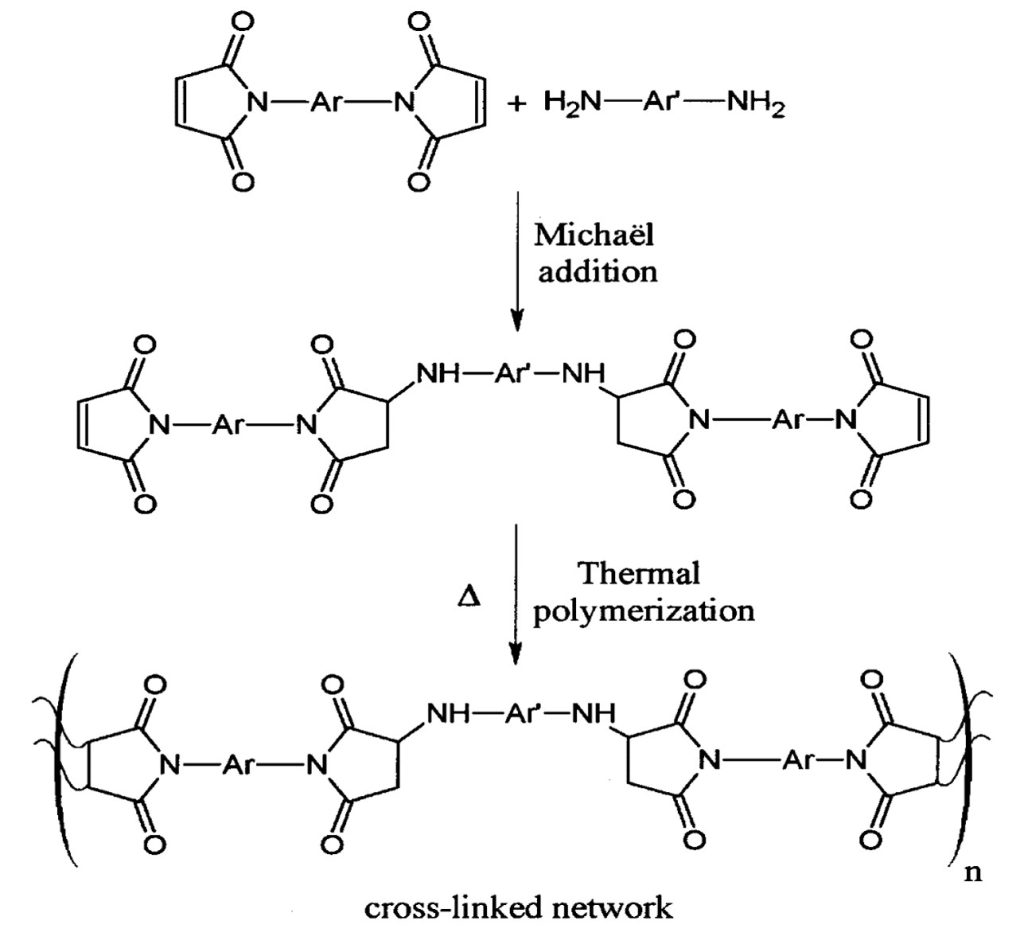
A common reaction pathway is called Michael addition. In this reaction scheme (shown in Figure 3), an amine containing molecule is reacted with the double bond on the maleimide group. Thermal polymerization of the maleimide double bonds does not lead to a highly crosslinked network due to steric hinderance as the aromatic bismaleimide chains react. To overcome the steric effects and lead to a high degree of crosslinking, Michael addition using amines has proven to be very useful [1-3].
Figure 3. Schematic of the Michael addition to BMI.
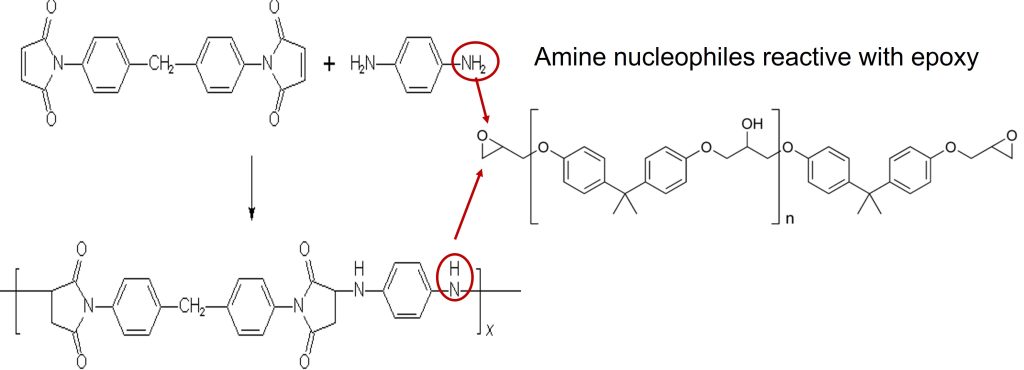
The choice of Ar and Ar’ in Figure 3 can lead to a wide range of crosslinked networks. Additionally, there are a wide range of commercially available amine compounds that can be use in a “molecular architect” approach to tailor the physical properties to achieve the desired final performance. A good example of this approach is shown in Figure 4.
Figure 4. Michael addition to bismaleimides in the presence of epoxy molecules.
In Figure 4, two formulation approaches are used to modify bismaleimides by chain extension via the Michael addition of the diamine and to co-react with epoxy to impart toughness. The formulation toolbox is large since there are a number of bismaleimides and amine functional hardeners. In Figure 4, I have noted where the amine nucleophiles (both primary amine (in the upper circle) and secondary amine (lower circle) are used to both react with the epoxy in a well-know nucleophilic addition reaction.
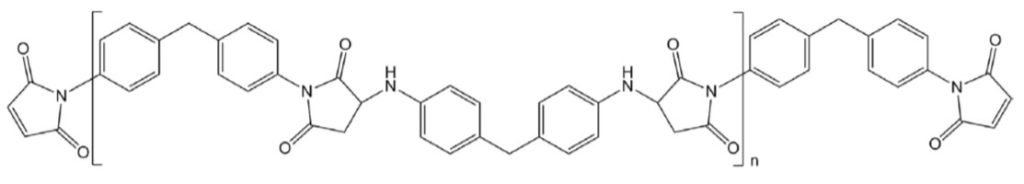
Two commercially available bismaleimides Kerimid 601 and COMPIMIDE® 200 use the Michael addition pathway to form the starting materials as shown in Figure 5.
Figure 5. Structure of commercial BMI resins KERIMID® 601 and COMPIMIDE® 200 [2, 4].
Readers who are epoxy formulators recognize the diamine linkage in the middle is from diamino diphenyl methane (DDM) typically used with four functional high performance epoxy resins TGDDM) for structural applications.
References 1 and 2 below are excellent sources for readers who would like a more detailed discussion of BMI chemistry and applications. For a more contemporary treatise, reference 2 by Ian Hammerton and coworkers covers “Modern Advances in Bismaleimide Resins Technology.”
References
- Horst Stenzenberger, BMI Resin Chemistry, ASM Handbook, Volume 21, Composites p. 97-104, 2001
- J. Iredale, C. Ward, and I. Hamerton, Modern Advances in Bismaleimide Resins Technology, Progress in Polymer Science, 69, (2017) p. 1-21
- Hamerton, High Perform. Polym. 8 (1996) 83–95
- KERIMID® 601 from Huntsman

WE ARE INTERESTED to use BMI resin and to produce a tough adhesive and resin for paint in order to use as varnish and coating for withstanding against of high temperature services like 200c in an acidic flow could you advise to me of any?