Guest Post by Dan Lamone
 UV-curable resins that utilize a cationic photopolymerization mechanism behave differently than the free-radical polymerizable resins that we discussed in the past two weeks. While cationic resins use UV light to initiate polymerization, these systems utilize completely different photoinitiators, monomeric materials and have their unique advantages and disadvantages in product and process selection. This week’s and next week’s posts will cover cationic systems in depth. Image courtesy of Dymax (www.dymax.com )
UV-curable resins that utilize a cationic photopolymerization mechanism behave differently than the free-radical polymerizable resins that we discussed in the past two weeks. While cationic resins use UV light to initiate polymerization, these systems utilize completely different photoinitiators, monomeric materials and have their unique advantages and disadvantages in product and process selection. This week’s and next week’s posts will cover cationic systems in depth. Image courtesy of Dymax (www.dymax.com )
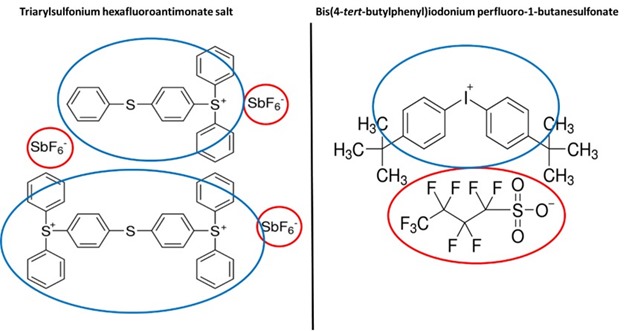
Cationic photoinitiators are also called photo-acid generators. Once a cationic photoinitiator absorbs UV radiation the initiator molecule is converted into a strong acid species, either a Lewis or Brönsted acid, that initiates polymerization. Most cationic photoinitiators are sulfonium or iodonium salts and as such are comprised of a cationic and anionic pair – each with a specific role in the reaction mechanism. The cationic portion of the photoinitiator molecule is responsible for the absorption of UV radiation while the anionic portion of the molecule becomes the strong acid after UV absorption. Example sulfonium and iodonium salts are shown below in Figure 1[1] with cationic portions labeled in blue and anionic portions labeled in red.
Figure 1. Two examples of classic cationic photoinitiator types: sulfonium and iodonium salts.
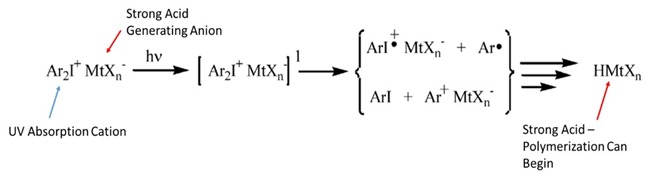
Just as different free-radical photoinitiators have different absorption spectra so do cationic photoinitiators based on different molecular configurations around the sulfur or iodine atom. The various anionic metal halides will produce strong acids of differing strength which correlates with reactivity rates (increased acid strength indicates increased reaction rates). The hexafluoroantimonate ion, one of the strong metal halide ions used in cationic photoinitiators, producing an pKa of ~ -30! Don’t be startled by the discussion of strong acids – the concentration of these materials in UV curable resins are in the hundredths of a percent range by weight. The reaction mechanism to produce a strong acid from a representative diaryliodonium salt molecule is shown below in Figure 2[2].
Figure 2. The cationic photoinitiator will absorb UV radiation, hv, to produce an excited electron state. The excited electron state caused the molecule to undergo both homolytic cleavage to produce several free radicals and heterolytic cleave to produce cationic species. The heterolytic products will react with monomers to stabilize themselves which in turn produces the strong acid.
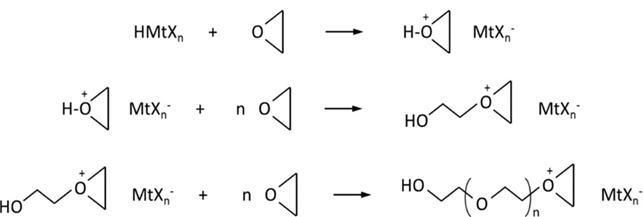
The cationic polymerization mechanism is consistent with chain-growth polymerization with initiation, propagation, termination and chain transfer steps. Whereas free-radical polymerization transfers a free-radical from monomer to monomer during chain growth, cationic polymerization transfers a charge from monomer to monomer during chain growth. Next week we will discuss monomeric materials used for cationic polymerization but the most common monomers are epoxide-based materials, specifically cycloaliphatic epoxies. For simplicity in Figure 3[3] below, the cyclic 3-member ring (ethylene oxide) is used to demonstrate the cationic polymerization mechanism for any epoxide based material. Step 1 is the protonation of an epoxy group from the photo-generated acid. Step 2 is the ring opening of the protonated epoxy group and charge transfer to an additional, new, epoxy group. Step 3 is the continued chain growth until all monomers are consumed, the charge is transferred to another chain or the reaction is terminated.
Figure 3. Cationic polymerization mechanism.
Next week we will discuss raw materials for UV-cationic resins and several unique implications of the cationic polymerization mechanism.
Sources
[1] Sigma Aldrich Photoinitiators. Accessed 2/1/2016. https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Aldrich/General_Information/photoinitiators.pdf
[2] Sangermano, M. 2012. Advances in cationic photopolymerization. Pure Appl. Chem. 84(10) 2089-2101.
[3] Vitale, A., Sangermano, M., Bongiovanni, R. Visible Light Curable Restorative Composites for Dental Applications Based on Epoxy Monomer. Materials. 7(1) 554-562

Hi Jeff : What a surprise to see your paper. How are you doing since Endicott ? I met a couple of years Bernd Appelt in Singapore. He retired in Florida. Cheers, marc