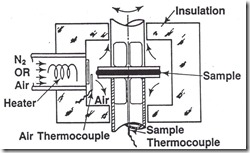
 Isothermal curing can be investigated nicely using oscillatory parallel plate rheometry. It is suggested that disposable plates be used to facilitate sample removal from the rheometer at the conclusion of the curing run. When setting up the rheometer, the strain amplitude needs to be determined. In most cases, a strain amplitude in the range of 0.1-0.3% is adequate to get good torque response over the viscosity range of interest for thermosets. Some rheometers have auto-strain control where the strain is larger for the lower viscosity and decreases as the resin cures. You will have to experiment with your particular instrument to dial in the right strain amplitudes and torque measurements.
Isothermal curing can be investigated nicely using oscillatory parallel plate rheometry. It is suggested that disposable plates be used to facilitate sample removal from the rheometer at the conclusion of the curing run. When setting up the rheometer, the strain amplitude needs to be determined. In most cases, a strain amplitude in the range of 0.1-0.3% is adequate to get good torque response over the viscosity range of interest for thermosets. Some rheometers have auto-strain control where the strain is larger for the lower viscosity and decreases as the resin cures. You will have to experiment with your particular instrument to dial in the right strain amplitudes and torque measurements.
One of the easiest cure experiments is to use the rheometer in the isothermal mode. In this case, the rheometer oven is pre-heated to the desired cure temperature and once the oven is equilibrated, the sample is quickly placed between the parallel plates. With some practice, the sample insertion process can be repeatable.
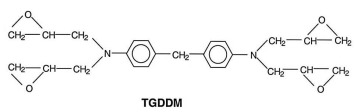
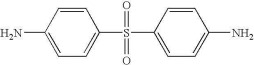
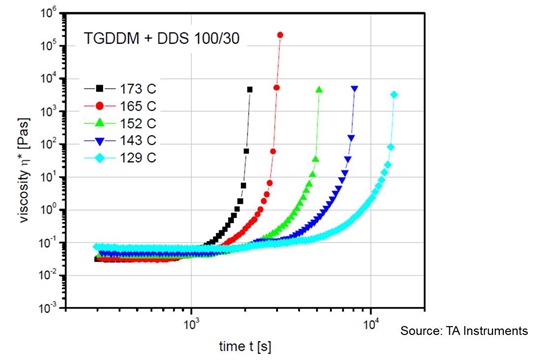
In the following figure, the complex viscosity is plotted as a function of time for a series of isothermal temperatures for a common structural epoxy (for example Araldite MY720). The epoxy resin is a tetrafunctional epoxy (TGDDM or tetra glycydl ether of diamino diphenyl methane) and the hardener is DDS (diamino diphenyl sulfone).
For the chemistry buffs in the crowd, the chemical structures are given below:
The epoxy resin is tetra functional along with a tetrafunctional amine and will lead to a highly crosslinked network rather quickly.
In the figure above one can see that the cure temperature has a profound influence on the viscosity profile. Remembering from our previous posts on gelation, one recalls that the viscosity diverges toward infinity at gelation. This represents the practical limit for flow in a molding or lamination process. The above plot is helpful in understanding the cure time dependence in that there is a quantitative gel time that can be estimated from the viscosity upturn.
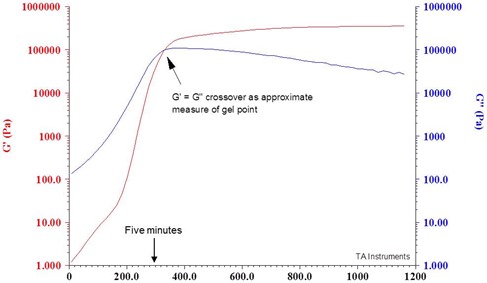
Let’s switch back to measurement of the gel point using rheometry. A classic way to estimate the gel time is to look at the dynamic moduli during isothermal curing. As discussed before, the gel point is the practical limit for processing. To look at this, we went to the local Home Depot (or Lowes) and got some Five Minute Epoxy. In the case of Five Minute Epoxy, the five minutes is the working time, or time the user has to get the parts in contact to ensure a good bond. From a molecular perspective, we wanted to see if the gel point occurred at five minutes. We mixed the Five Minute Epoxy according to the manufacturers instructions and quickly placed the mixed epoxy into the rheometer at room temperature. The following graph shows G’ and G” as a function of time.
As observed in our last post, early in the cure G” is the major contributor and G’ has a small impact. We measured the point were G’=G” and determined that the crossover occurs right around 5 minutes. So remember when you use Five Minute Epoxy, you better get your parts joined quickly or gelation will rear it’s ugly head!
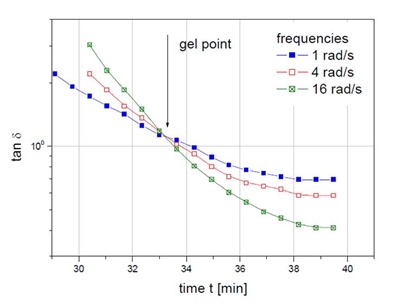
Professor Henning Winter at University of Massachusetts demonstrated that at the gel point, tan delta is frequency independent (1-3). To show that, in the following figure tan delta is plotted as a function of time for three frequencies (from TA Instruments).
One clearly observes that tan delta is frequency independent at gelation. When developing a thermosetting process, such as lamination, resin transfer molding, or autoclave molding, conducting some careful rheology experiments will provide insights into how to set the temperatures to achieve the optimal process.
- H. H. Winter et. al., Journal of Rheology, v. 30, p. 367-382, 1986
- H. H. Winter et. al., Journal of Rheology, v. 31, p. 683-697, 1987
- H. H. Winter et. al., Polymer Bulletin, v. 13, p. 499-503, 1985

Leave a Reply