 In the series on epoxy cure chemistry we covered epoxies, curing agents, and the chemical crosslinking pathways (nucleophilic reactions). In this post we will discuss how to put all the pieces together to do some “molecular engineering” to formulate products with different fully cured properties. This type of approach has been used extensively in commercial products from adhesives, to laminates to structural composites.
In the series on epoxy cure chemistry we covered epoxies, curing agents, and the chemical crosslinking pathways (nucleophilic reactions). In this post we will discuss how to put all the pieces together to do some “molecular engineering” to formulate products with different fully cured properties. This type of approach has been used extensively in commercial products from adhesives, to laminates to structural composites.
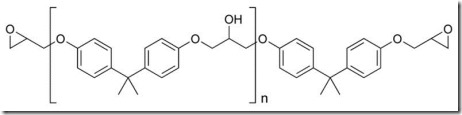
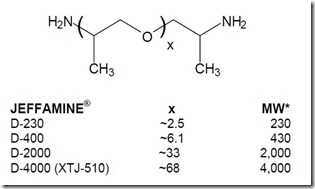
Let’s recall that epoxies are supplied in a wide range of molecular weights (n can vary in the chemical structure below) and amine curing agents also can have various molecular weights as well as seen in the figure showing the various types of Jeffamine (1) curing agents.
Let’s look at a few different molecular architectures. In the following examples we assume that the epoxy functionality is two and the amine functionality is four (remember primary amines can react with two epoxy groups).
The first case is where the molecular weights between the crosslinks is higher at approximately 1000 (for example EPON 1001F or DER 671 see references 2 and 3))
In this case the final cured network will have LOWER
Glass transition temperature (Tg)
Modulus
End use temperatures
but have HIGHER
Toughness
Flexibility
In the second case the molecular weight between crosslinks is smaller at approximately 380 (for example EPON 828 or DER 331, see references 2 and 3))
In this case the final cured network will have HIGHER
Glass transition temperature (Tg)
Modulus
End use temperatures
Solvent resistance
but have LOWER
Fracture toughness
Flexibility (more brittle)
In the above two examples, we kept the amine constant and showed the effect of changing the epoxy chain length between crosslinks. One could envision even more customization by using longer or shorter amine curing agents such as the Jeffamine series above. For example, using a long chain epoxy (EPON 1007F, MW is approximately 2900) and a long chain diamine (Jeffamine D-2000, MW is approximately 2000) would lead to a very low Tg network that is tough and highly elastic.
In structural composite applications, the goal is to have high Tg, high modulus and stiffness, good resistance to chemicals, and high end use temperatures. A common epoxy formulation used a four functional epoxy and an aromatic diamine.
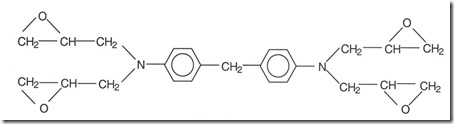
The figure below shows the chemical structure of 4,4′-diaminodiphenylmethane (TGDDM) such as MY 720 and MY 721 from Huntsman:
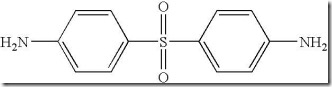
and 4,4′-diaminodiphenylsulfone(DDS):
Due to the highly aromatic structures and the functionality of each molecule is four, then a very tightly crosslinked, high modulus network will result. One drawback of the highly crosslinked network is low fracture toughness, but there are many ways to improve the fracture toughness.
References:
1) Jeffamines are trademarks for amine curing agents provided by Huntsman Performance Products. Click for detailed product information
2) Momentive Performance Materials. click for more details
3) Dow Chemical. click for more details

Very informative. How can I reach you for a further discussion