Guest Post by Dr. Robert Humphreys
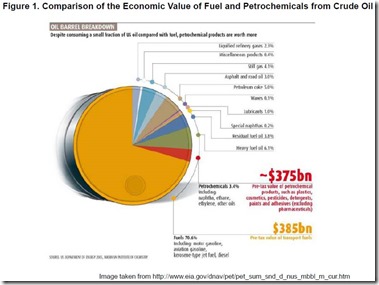
 Consumers have grown accustomed to and even dependent on a superabundance of desirable, affordable products and technologies that address their every need for nutrition, comfort, shelter, health, pleasure, information, communication, transportation, education, and intellectual stimulation. On the other hand, it is the rare person who understands that this bounty would be impossible without abundant, affordable raw materials produced by a petrochemicals industry that, in turn, derives much of its feedstock from a comparatively much larger petroleum-based fuels industry. The relative economic value of petroleum-based chemicals compared to fuels is illustrated in Figure 1. This chart alone should be enough to explain why many companies working on lignocellulosic biomass (LB) as a renewable raw material are developing renewable versions of petrochemicals rather than biofuels and why we chose to focus the 6th post in this series on conversion of pretreated LB to renewable chemicals and polymers rather than biofuels.
Consumers have grown accustomed to and even dependent on a superabundance of desirable, affordable products and technologies that address their every need for nutrition, comfort, shelter, health, pleasure, information, communication, transportation, education, and intellectual stimulation. On the other hand, it is the rare person who understands that this bounty would be impossible without abundant, affordable raw materials produced by a petrochemicals industry that, in turn, derives much of its feedstock from a comparatively much larger petroleum-based fuels industry. The relative economic value of petroleum-based chemicals compared to fuels is illustrated in Figure 1. This chart alone should be enough to explain why many companies working on lignocellulosic biomass (LB) as a renewable raw material are developing renewable versions of petrochemicals rather than biofuels and why we chose to focus the 6th post in this series on conversion of pretreated LB to renewable chemicals and polymers rather than biofuels.
Technologies for Converting Pretreated LB into Renewable Chemicals and Polymers
Our taxonomy for conversion of LB to renewable chemicals and polymers is based on the type of conversion process employed:
- Biological conversion
- Thermal conversion
- Chemical conversion
- Direct separation or extraction from a plant or organism.
These conversion processes involve very different conditions and result in dramatically different types of products, so we will provide some detail on 1-3. We will devote the final post in this series to conversion process 4, since it is largely dependent on technology that has been practiced for decades or even centuries.
Biological Conversion
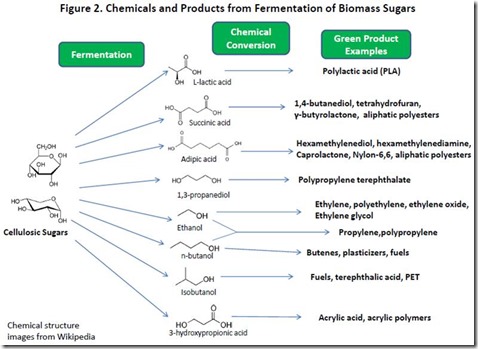
Mixtures of C6 and C5 sugars from the cellulose and hemicellulose fractions of LB (see Conversion to Sugars in Biomass pretreatment) are converted to a target metabolite by fermentation using an organism (most often S. cerevisiae or E. coli) engineered to optimize conversion of sugars to the metabolite. The target metabolite is typically a small molecule (eg. lactic acid, succinic acid, adipic acid, 1,3-propanediol, ethanol, n-butanol, isobutanol, 3-hydroxypropionic acid). These metabolites also can be converted into higher value molecules and polymers using chemical and catalytic processes. Some examples of products from chemical conversion of these fermentation chemicals are shown in Figure 2.
Thermal Conversion
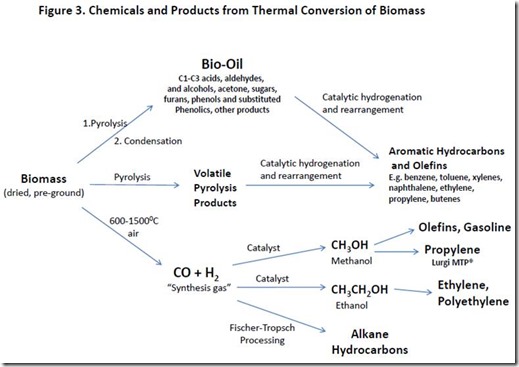
Pretreated biomass (see Mechanical Pretreatments in Biomass pretreatment) is pyrolized (heated to high enough temperature, typically >4500C, that extensive chemical bond breakage occurs) in the absence of oxygen. The gases that are formed can be condensed and collected as pyrolysis oil (also called bio-oil, see Cracking into Bio-oil in Bio-oil) or introduced into additional reactors for further processing in the presence of catalysts and hydrogen. Pyrolysis oil also can be processed further in separate reactors in the presence of catalysts and hydrogen. Pyrolysis is applied to production of fuels and chemicals (see page 4068-4071 in Fuels from biomass/Huber). Production of fuels and chemicals with a lower ratio of oxygen to carbon than biomass or sugar feedstock requires a source of hydrogen gas, which can be from petroleum, natural gas or biomass gasification.
Biomass gasification involves heating dry biomass in a reactor at high temperature (600-15000C) with dry air. The amount of air used is enough to result in incomplete combustion of the biomass, producing a mixture of CO and H2 (“synthesis gas”) as well as CO2, H2O, methane (CH4), and N2 (see Synthesis gas from biomass gasification). Synthesis gas can be used to produce products such as methanol and ethanol or can be converted to hydrocarbon fuels using the Fischer-Tropsch process. Methanol, in turn, can be converted into propylene using the Lurgi MTP® process (see Lurgi MTP) and ethanol can be converted to products as shown in Figure 2).
Some examples of products from thermal conversion of biomass are shown in Figure 3.
Chemical Conversion
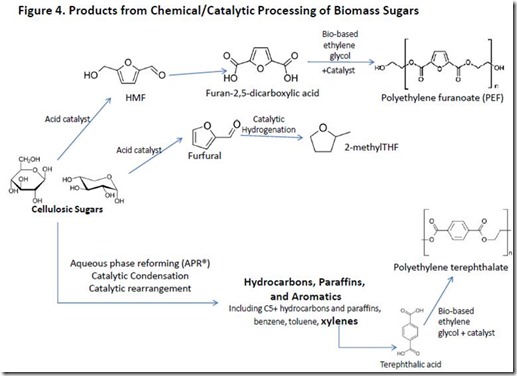
C6 and C5 sugars can be converted chemically into commercial molecules. For example, treatment of glucose or fructose with various acid catalysts generates 5-hydroxymethylfurfural (HMF) and, with extended treatment, levulinic acid. HMF can be oxidized efficiently to produce 2,5-furandicarboxylic acid, a monomer component of the novel, renewable polyester, polyethylene furanoate, or PEF, that is a potential replacement for petroleum-based PET polyester (see PEF). The C5 sugar, xylose, is converted into furfural, which can be converted to the important solvent 2-methyltetrahydrofuran (2-methylTHF).
Chemical conversion can also be accomplished by catalytic reforming of sugars in water at elevated temperature and pressure (180-2200C, 10-90 bar) followed by treatment with catalysts that favor classical Aldol-type condensation chemistry and rearrangement or aromatic ring formation (see, for example, catalytic conversion of sugars, and US Patents 7,977,517B2 and 8,017,818B2). This type of processing can produce liquid fuels or mixtures of aromatic hydrocarbons, depending on conditions and catalysts.
Examples of products made by various combinations of chemical and catalytic processing of biomass sugars are shown in Figure 4.
Moving On to the Real World of Biomass Processing
We don’t mean this subtitle to be facetious. In the real world of bulk or specialty chemicals or fuels, biomass conversions will need to utilize a range of biomass types and quality coming from the field via the logistical systems described in a previous post in this series (see Biomass logistics and Biomass feedstocks). Technologies that convert this biomass most efficiently to products that customers want (i.e. will pay for) will have the best chance of commercial success. The environment in which this saga will play out is evolving continually, including the rules of engagement and the competition (e.g. Is a carbon tax inevitable and if so, how much and when? How long will it really take to establish a non-food biomass infrastructure at scale? What will the longer term impact of shale gas and oil be on “energy independence” and the development of biofuels and renewable chemicals?). It will be riveting to watch. The final post in this series will focus on renewable chemicals and polymers that have been a part of human technology for decades and even centuries.

Leave a Reply