Guest Post by Dr. Robert Humphreys
 In the previous post, Dr. Gotro presented some amazing data on production of bio-ethylene (bio-E) and bio-polyethylene (bio-PE). It may come as a surprise to find that fermentation, the process that is used to make beer, wine, yogurt and cheese, is also the source of bio-ethylene that is polymerized to bio-PE. In this post, we will give a short overview of how fermentation converts commercially important sugars (glucose from starch or cellulose; glucose and fructose from sucrose, often referred to as table sugar) into ethanol and how ethanol is converted to bio-E, which is the starting monomer for bio-based polyethylene (bio-PE).
In the previous post, Dr. Gotro presented some amazing data on production of bio-ethylene (bio-E) and bio-polyethylene (bio-PE). It may come as a surprise to find that fermentation, the process that is used to make beer, wine, yogurt and cheese, is also the source of bio-ethylene that is polymerized to bio-PE. In this post, we will give a short overview of how fermentation converts commercially important sugars (glucose from starch or cellulose; glucose and fructose from sucrose, often referred to as table sugar) into ethanol and how ethanol is converted to bio-E, which is the starting monomer for bio-based polyethylene (bio-PE).
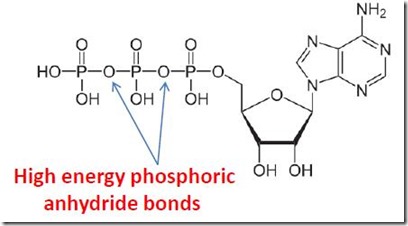
Fermentation is the anaerobic (= occurring in the absence of oxygen) metabolic process by which living organisms convert food sources, such as simple sugars, into ATP (adenosine triphosphate) [1]. ATP has been called the universal energy currency for life on earth and is the principal source of stored chemical energy used by all organisms to power the chemical processes of life. For some simple organisms, fermentation is the only source of ATP. Animals including humans rely on respiration for ATP production, an oxygen-dependent process that is much more efficient than fermentation. Nevertheless, animals use fermentation for production of ATP when oxygen availability is very limited (“hypoxia”), such as in muscle tissue during vigorous exercise when oxygen supply cannot keep up with demand.
Figure 1. ATP, or Adenosine Triphosphate, the source of stored chemical energy used for all of the other processes of life.
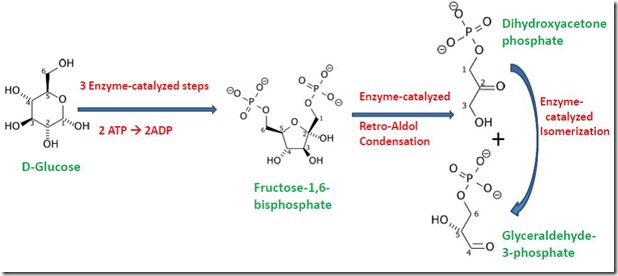
Biochemically, fermentation can be divided into two parts. The first is glycolysis. Glycolysis occurs via multiple enzyme-catalyzed steps that degrade glucose into two molecules of pyruvate, simultaneously generating two molecules of ATP. Glycolysis occurs in two phases. The first is the Preparatory Phase, shown in Figure 2. The Preparatory Phase involves five enzyme-catalyzed steps that convert glucose into molecules of glyceraldehyde 3-phosphate, including the final enzyme-catalyzed reaction that isomerizes one molecule of dihydroxyacetone phosphate to glyceraldehyde 3-phosphate. This means the Preparatory Phase converts the 6-carbon glucose molecule to two 3-carbon molecules of glyceraldehyde 3-phosphate. Note that the glucose skeleton is isomerized to fructose during the process, so it should not be surprising to find that fructose also undergoes glycolysis. Also, the Preparatory Phase uses two ATP’s to generate fructose-1,6-bisphosophate as an intermediate, which means the Preparatory Phase actually consumes two molecules of ATP instead of generating ATP!
Figure 2. The Preparatory Phase of Glycolysis.
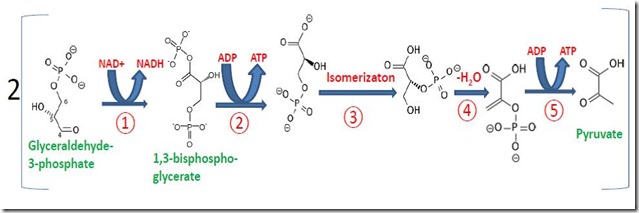
The second phase of glycolysis is called the Payoff Phase because it is generates more ATP than the Preparatory Phase consumes, which is the energy “payoff” for the organism. Figure 3 shows that each glyceraldehyde 3-phosphate is converted to two molecules of pyruvate in five enzyme-catalyzed steps, a transformation that produces two molecules of ATP (Steps ② and ⑤). Since one glucose molecule generates two molecules of glyceraldehyde 3-phosphate, the Payoff Phase produces four molecules of ATP. So, the Preparatory Phase consumes two ATP’s and the Payoff Phase generates four ATP’s, for an overall net of two molecules of ATP from glycolysis of glucose (or fructose). This ATP is available for the organism to use as a chemical energy source to power all of the other processes of life.
Figure 3. The Payoff Phase of Glycolysis
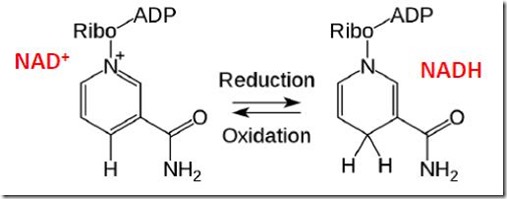
Pyruvate is a more oxidized molecule than glyceraldehyde-3-phosphate. The oxidation occurs in Step ① and requires nicotinamide adenine dinucleotide (NAD+), a “co-enzyme” that accepts and donates electrons during enzyme-catalyzed oxidations and reductions (Figure 4). NAD+ is reduced to NADH as glyceraldehyde-3-phosphate is oxidized in Step ①. This leaves the organism with a problem: it must find a way to convert NADH back to NAD+ so it can be recycled continuously in glycolysis. NAD+ is a complex molecule, as is ATP, which means it is necessary to recycle these molecules continuously since re-synthesizing them would be energetically prohibitive. How does the organism accomplish this recycling?
Figure 4. Oxidized (NAD+) and Reduced (NADH) Forms of Nicotinamide Adenine Dinucleotide
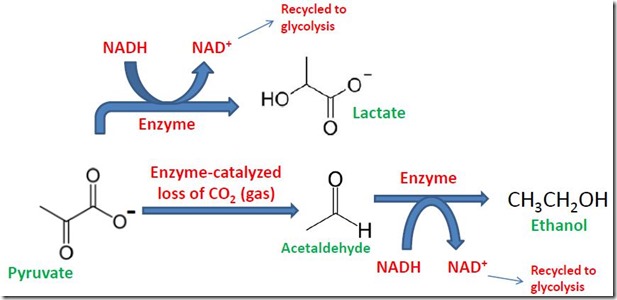
The answer is provided in the second part of fermentation (Figure 5). NAD+ is oxidized back to NADH by reducing pyruvate to a fermentation product. When ethanol is the fermentation product, an enzyme-catalyzed reaction causes pyruvate to lose carbon dioxide to form acetaldehyde [2]. Acetaldehyde is then reduced to ethanol by NADH, simultaneously regenerating NAD+ that can be recycled to glycolysis. This is the process that occurs during yeast-based fermentation to make wine and beer and also causes the rising of bread dough. Other organisms regenerate NAD+ by other processes, such as reduction of pyruvate to lactate (also shown in Figure 5). Dr. Gotro has previously reported on PLA, or polylactic acid, a commercially important bio-plastic that is produced from lactic acid derived from fermentation of glucose (see Sept. 17 and 24, 2012 posts, this site).
Figure 5. Recycle of NAD+ by Reduction of Pyruvate to Ethanol or Lactate
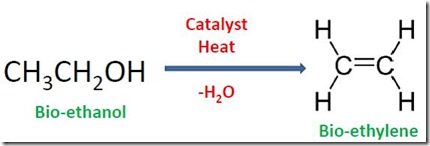
Finally, fermentation-derived ethanol can be dehydrated to bio-ethylene at elevated temperature (>3000C.) using a variety of catalysts (Figure 6). SynDol® (based on MgO-Al2O3/SiO2) is a type of heterogeneous (i.e. used as a solid) catalyst that is used commercially [3]. Bio-ethylene is polymerized to bio-PE employing typical industrial polyethylene technology.
Figure 6. Dehydration of Bio-Ethanol to Bio-Ethylene
In this post Dr. Humphreys covered a lot of the basic chemistry used in many bioplastics. This post gives the reader a solid foundation in the fermentation process used in many biopolymers.
[1] For more detail on glycolysis and fermentation biochemistry, see: a) “Lehinnger: Principles of Biochemistry”, 4th ed., David L. Nelson and Michael M. Cox, W.H.Freeman and Company, New York, 2005; b) “Molecular Cell Biology”, H. Lodish, A. Beck, C.A.Kaiser, M. Krieger, M.P.Scott, A. Bretscher, H. Ploegh, and P. Matsudaira, W.H.Freeman and Company, New York, 2007.
[2] Note that the carbon dioxide is lost as a gas. This reduces the “carbon efficiency” of the ethanol fermentation process to 2/3, or about 67% since two glucose carbons are lost to produce two ethanol molecules. On a weight basis, the efficiency is only about 34% since the molecular weight of ethanol is only 46.07 g/mol. These numbers assume quantitative conversion, which is never attained.
[3] For some references on ethanol dehydration, see: a) “Ethylene formation by catalytic dehydration of ethanol: industrial considerations”, Denise Fan, Der-Jong Dai, and Ho-Shing Wu, Materials, 6, 101-115 (2013); b) “Catalytic dehydration of bioethanol to ethylene over TiO2/γ-Al2O3 catalysts in microchannel reactors”, G. Chen, S. Li, F. Jiao, and Q. Yuan, Catalysis Today, 125, 111-119(2007)

Leave a Reply