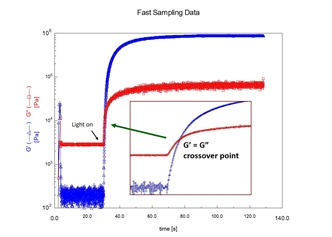
 In the last post the UV rheometer method was found to be a very sensitive measurement tool for determining the gel point during UV curing. As seen in the figure on the left, the crossover in the dynamic loss and storage moduli can be precisely determined yielding an approximate measurement of the time to gelation. The time to gelation is an important parameter in the processing of thermosetting resin formulations.
In the last post the UV rheometer method was found to be a very sensitive measurement tool for determining the gel point during UV curing. As seen in the figure on the left, the crossover in the dynamic loss and storage moduli can be precisely determined yielding an approximate measurement of the time to gelation. The time to gelation is an important parameter in the processing of thermosetting resin formulations.
In this post, the role of UV intensity and UV dosage will be explored. As in the previous post the data presented here is from a very good TA instruments application note (1). First, let’s investigate the role of UV intensity on the curing rate.
Figure Courtesy of TA Instruments
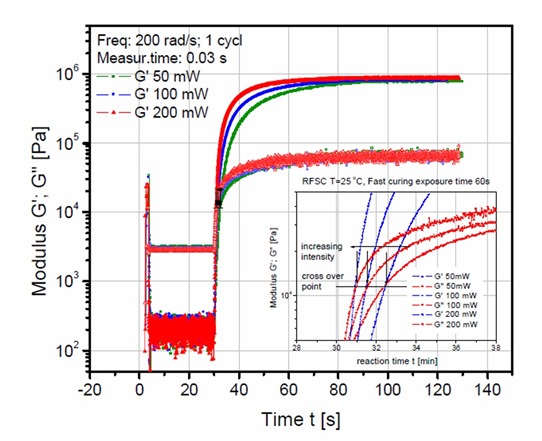
Figure 1. Dynamic moduli, G’ and G” as a function of time for various UV exposure doses
In Figure 1, the UV intensity was varied from 50 mW to 200mW. The effect of intensity was investigated by exposing the sample in the rheometer for a constant time (60 seconds) at three levels of intensity. The highest intensity (200mW – red curve in Figure 1) shows the fastest curing rate, the the curing rate decreasing as the UV intensity decreases. In most of these free radical type of UV curing systems, the reaction rate depends on the number of free radicals in the system. On UV exposure, typical photoinitiators produce free radicals which in turn initiate crosslinking. Thus, as demonstrated in Figure 1, the higher the UV intensity, the faster the reaction rate. By carefully measuring the slope of the complex modulus versus time and plotting as a function of UV intensity, a straight line relationship was found (1). In Figure 2, the region of the dynamic moduli crossover has been expanded.
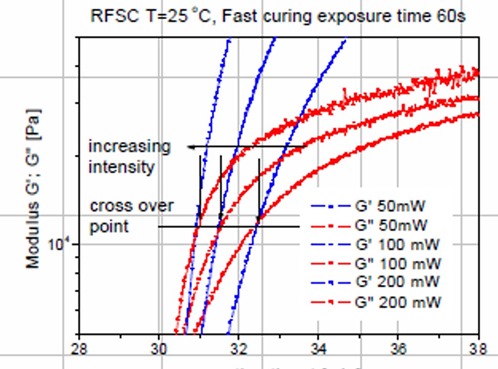 Figure Courtesy of TA Instruments
Figure Courtesy of TA Instruments
Figure 2. Expanded section near the dynamic moduli crossover for the dynamic moduli, G’ and G” as a function of time for various UV exposure doses.
In Figure 2, the blue curves are the dynamic storage moduli (G’) and the red curves are the dynamic loss moduli (G”) as a function of reaction time for three UV doses. The G’ = G” crossover point is noted by a horizontal line in Figure 2. There are several very interesting features to note. First, as the UV intensity increases, the time to the dynamic moduli crossover decreases (that is increasing the UV intensity causes faster UV induced polymerization resulting in a faster time to gelation). This is exactly what one would expect since for a fixed amount of UV initiator, the higher the UV intensity, the larger the number of free radicals that would be generated.
The second interesting feature in Figure 2 is that the G’=G” crossover occurs at nearly the same modulus (denoted by the horizontal line). It is well established that the conversion at the gel point is determined solely by the chemical composition. The conversion at the gel point can also be calculated knowing the types and concentrations of the reacting species. With this in mind, since the conversion at the gel point is defined by the chemistry alone, the gel point is also called a iso-conversion point. Note that in Figure 2, the dynamic moduli crossover occurs at approximately the same modulus, i.e. an iso-conversional point further demonstrating the utility of the UV rheometer method for precise characterization of the physical changes during UV curing.
References
1) TA Instruments Applications Note AAN021

Leave a Reply