Guest Post by Dan Lamone
Many commercial systems use UV exposure to initiate curing as seen in the image above (courtesy of Dymax, www.dymax.com ) Free radical generating photoinitiators are used to cure through double bonds, most commonly acrylate and methacrylate monomers or oligomers. There are two types of free-radical generating photoinitiators, designated as Type I and Type II photoinitiators.
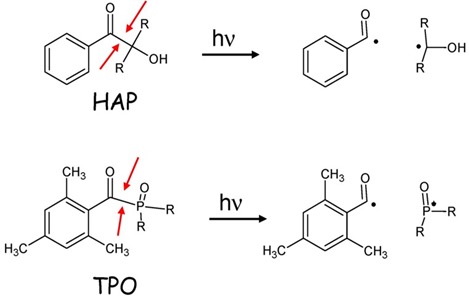
Type I photoinitiators are unimolecular free-radical generators; that is upon the absorption of UV-light a specific bond within the initiator’s structure undergoes homolytic cleavage to produce free radicals. Homolytic cleavage is a bonding pair of electron’s even scission into to free radical products. Shown below in Figure 1 are two examples of homolytic cleavage in two common classes of Type I photoinitiators: hydroxyacetophenone (HAP) and phosphineoxide (TPO) [1]
Figure 1. The highlighted bond in each species undergoes homolytic cleavage upon the absorption of UV light. HAP’s usually absorb shorter wavelength UV-A radiation (300-350nm) while TPO’s commonly absorb radiation in the 360-400nm range, nearly into visible light.
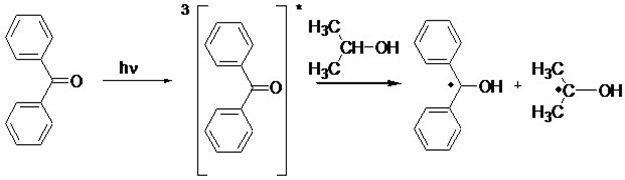
Type II photoinitiators require a co-initiator, usually an alcohol or amine, functional groups that can readily have hydrogens abstracted, in addition to the photoinitiator. The absorption of UV light by a Type-II photoinitiator causes an excited electron state in the photoinitiator that will abstract a hydrogen from the co-initiator, and in the process, splitting a bonding pair of electrons. An example mechanism is shown in Figure 2[2].
Figure 2. Benzophenone and benzophenone-type photoinitiators are the most common Type II photoinitiators. Most Type II initiators absorb in the UV-C region.
Once the free-radicals are generated, the polymerization mechanism is no different than any free-radical polymerization process. The chain propagation, termination and transfer steps found in other free-radical processes are consistent in UV polymerization. Mechanistically, it is only the initiation process to generate free radicals that separates UV polymerization apart. It is this initiation process however that gives UV-curable resins, specifically free-radical UV-curable resins, a very unique advantage over traditional free-radical systems and that advantage is control.
In UV polymerization process you have complete control over the number and rate of free-radicals generated at any point in the process. Free radicals can only be generated upon absorption of UV radiation and the source of UV radiation is defined and controlled by your process (an important subject to be discussed in a later post). If you are familiar with thermally-activated free-radical initiators, usually azo or peroxide-based initiators, you will recognize the initiator half-lives as a function of temperature provided by the manufacturer. This half-life generally indicates the temperature at which half of the initiator molecules split to generate free-radicals with in a specific time period, usually one hour. What this relationship implicitly implies is that at all temperatures free-radicals are generated, with lower rates of generation at low temperatures and higher rates of generation at higher temperatures – generally following the Arrhenius relationship (i.e. a 2x increase in reaction rate every 10°C). It is this reason that thermally-activated, free-radical initiated systems have specific storage requirements (often below 0°C), pot-lives, and require specific oven/thermal profiles. While UV polymerizable resins have specific UV exposure requirements, they do not have pot-lives or freezer storage requirements – they are stable at room temperature in the absence of UV light.
In our next post we will discuss the monomeric and oligomeric materials that utilized free-radical photoinitiators.
[1] Fouassier JP, Lalevée J. 2014. Photochemical Production of Interpenetrating Polymer Networks; Simultaneous Initiation of Radical and Cationic Polymerization Reactions. Polymers. 6(10), 2588‑2610
[2] YAGCI Polymer Research Group. Photopolymerization. Accessed 1/17/2016. https://web.itu.edu.tr/~yusuf/photopolymerization.htm


hello
I am a PhD student. My thesis is defined base on acrylated Polyurethan. I used HEMA (hydroxy ethylmethacrylate) as a reactive diluent and benzophenone (BP) as a UV initiator with methyl diethanolamine as an accelator. But my system is so viscous and needs heating for adding photo initiator and proper mixing. I use 45º c for adding photo initiator and mixing. but most of the time it stat to crosslinking from the bottom of the sample container. I mean before curing with UV lamp it start crosslinking.
I thought that its because of BP . Its a thermal initiator too. so I need a UV initiator can be used in higher temperature. Could you please introduce appropriate one?
Dear All,
I want to make UV resin based paste which can be cured with in 30 second. For that I have used acrylate UV resin in liquid form. So what should I mixed in powder form which does not effect curing time and the curing depth up to 1/2″. I think UV absorver with photo stabilizer and glass powder can work well. Please suggest me for this.
. My working assumption has been that free radical kinetics dictate the efficiency of the polymerization reaction in turn influencing polymer chain length, quality of the reaction product and measurable polymer characteristics. Free radical kinetics in many reactions are limited by recombination. Does that limitation apply to polymerization? Thanks.