Guest Post by Dan Lamone
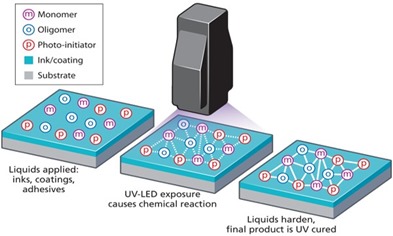 Image courtesy of LED Magazine
Image courtesy of LED Magazine
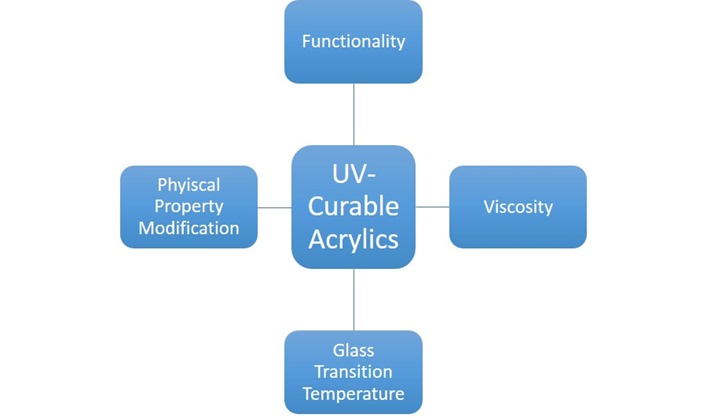
Product performance for free-radical curing UV resins can be tailored to meet many customer requirements due to the wide range of monomeric and oligomeric materials available. Most free-radical curing UV resins employ the unsaturated functionality of acrylate (including methacrylates) for curing but allyl and vinyl-based formulations exist as well. For this post, we keep our discussion focused around acrylate-based materials. Thankfully chemists and chemical engineers have devised ways to add acrylic functionality to almost any molecule. Whether the desired base molecule is a small molecule or high molecular weight oligomer, it is highly likely that an acrylated version exists commercially. The graphic below demonstrates several dimensions a formulator or product development engineer can utilize the wide range of acrylic monomers to design a UV curable resin.
Monomeric functionality is related to the degree of crosslinking within your polymer system. Mono-functional and di-functional materials are often used to balance flow and internal strength within adhesive and coatings applications. Di and tri functional raw materials find use in many coatings applications. Higher functional materials with 4,5,6, up to 18 reactive sites add additional cross-linking into the resin, often imparting additional chemical and mechanical resistance. Monomeric materials will typically have molecular weights less than 500 while oligomeric materials could have molecular weights into the tens of thousands. For acrylic systems, always keep in mind that higher degrees of crosslinking translate to higher shrinkage rates in the cured material – for this reason, many formulations will use blends of mono-functional monomers and both higher functional monomers and oligomers.
The application viscosity for UV-curable coatings can be tuned to match your application. Water-thin mono-functional monomers such as methyl methacrylate, isobornyl acrylate and isodecyl acrylate and higher functional monomers such as hexanediol diacrylate and trimethylolpropane triacrylate can be used as reactive diluents to cut resin viscosities. Mono-functional acrylic resins are typically more effective in reducing resin viscosity but those applications requiring higher degrees of cross-linking can use higher functional monomers as reactive diluents. Oligomer materials span a large viscosity range with viscosities starting as low as 100cP all the way up to several million cP.
What polymer properties are desired in your UV-curable resin? High modulus? Soft, flexible? High chemical resistance? Specific compatibility to a coated surface? Another benefit of using an acrylated oligomer is that the backbone of the oligomer can be of a different polymer class: highly-flexible polybutadienes, rigid and chemically-resistance bisphenol A epoxies, and flexible yet tough polyurethanes. Polyester and acrylic-based acrylate oligomers are also commercially available.
The glass transition temperature, related to the end-use temperature, for an UV-curable resin be tuned over a wide range of temperatures. Monomeric glass transition temperatures can range from -60°C, isodecyl acrylate, to 110°C, isobornyl and methyl methacrylate. Be mindful of your polymerization conditions when using acrylic materials with glass-transition temperatures significantly over room temperature, say greater than 45°C. Most acrylics are cured at or near room temperature with additional heating from the heat of reaction and thermal energy of the UV source itself as factors to consider. Vitrification, a subject of many posts here on Polymer Innovation Blog, should be a concern if the glass transition temperature of your UV-curable resin is higher than room temperature and your process does not directly account for this temperature discrepancy.
My intent for this post was to be a little top-heavy on the chemistry. As you’ve just seen, acrylate-based UV-curable resins can be tailored to a wide range of applications. Unlike other polymerization technologies where process variables can be used to tune end-use properties, such as reaction temperature to the change molecular weight distribution, most properties of UV-curable resins are derived from the base molecules themselves and not the polymerization process.
In our next post we will discuss another type of UV-curable photoinitiators, cationic and anionic photoinitiators.
Extra Credit: Overcoming Oxygen Inhibition in UV-Curable Acrylics
While UV-curable acrylics are highly flexible in product development, they do have their own challenges in production, most notably, as with all free-radical reactions with acrylics, the polymerization hindrance of oxygen. While free-radicals are a highly-energetic reactive intermediates that will react with just about any organic material to find stable-bonding partner, they do have a preference for the electron-dense oxygen molecule when it is available. The peroxy-radical generated by the reaction between a free-radical and an oxygen is a meta-stable reaction product than exist in solution for extended periods of time as the peroxy-radical is somewhat stable.
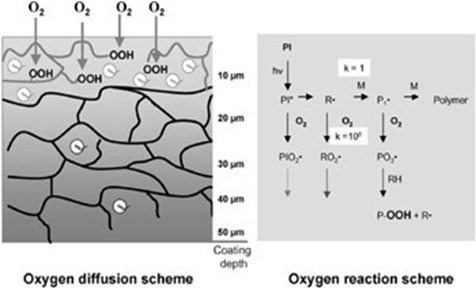 Image courtesy of Reinhold Schwalm
Image courtesy of Reinhold Schwalm
All acrylates come with an inhibitor, usually a quinone-based compound, that reacts with peroxy-radicals and terminates any possible polymerization reaction, stabilizing acrylic products in storage against spontaneous polymerization which can occur in uninhibited acrylates. The inhibitors are just as active in storage as they are in your process and therefore you process must account for the inhibitors presence – especially in thin-film materials. Several options are at your disposal with the most common being: oxygen removal, amine synergists, and perhaps my favorite, brute-force polymerization.
- Curing under an inert, usually nitrogen, atmosphere; no oxygen, no peroxy-radical, no inhibition. The inhibitor remains the material unused and along for the ride.
- Amine synergists offer two benefits, hydrogen donation for Type II free-radical photoinitiators and conversion of peroxy-radicals into reactive species that do not react with inhibitors, rendering the inhibitors moot and allowing polymerization to occur unencumbered.
- The equilibrium concentration of oxygen of acrylates is low, often below 50ppm and free-radical initiator concentrations are often well inhibitor concentrations. If the rate of polymerization exceeds the rate oxygen can diffuse into your material, it is possible to simply overcome the effect the oxygen. As your material polymerizations, viscosity rapidly increases and at the surface can “skin-over” retarding oxygen’s ability to diffuse into your materials. The inhibitor will terminate polymerization of the initial free-radicals but once consumed, polymerization will be unaffected. UV processes utilizing high-power sources or materials with high-crosslink densities often use this technique.
Bill Cortelyou. UV-LED advancements extend the promise in curing. LED Magazine. Published March 11, 2014. https://www.ledsmagazine.com/articles/print/volume-11/issue-3/features/science/uv-led-advancements-extend-the-promise-in-curing.html. Accessed 1/25/2015
Reinhold Schwalm. Chapter 7: Tackling the Drawbacks of UV Systems. https://www.globalspec.com/reference/40251/203279/chapter-7-tackling-the-drawbacks-of-uv-systems. Accessed 1/26/2015

Leave a Reply