Guest Post by Dan Lamone
 We can classify portions of the electromagnetic spectrum by designating the frequency OR wavelength range of electromagnetic radiation because of wonderfully simple equation: c = f * λ. The speed of light (c), a constant, is equal to the product of frequency (f) and wavelength (λ). Once a wavelength is designated we automatically know the radiation’s frequency, as well as several other characteristics, namely photon energy. We can also classify regions of the electromagnetic spectrum by the interaction of electromagnetic radiation with matter. For example, microwave radiation interacts with matter by causing molecular rotation and vibration (heating up a cup of tea) while x-ray radiation possesses enough energy to ionize atoms (a medical CT scan). Ultraviolet (UV) radiation is a region of the electromagnetic spectrum that interacts with and excites atomic and molecular valence electrons, the electrons involved in chemical reactions. Indeed, the original literature on UV radiation, circa 1800-1850’s, referred to these light rays as chemical or oxidizing rays.
We can classify portions of the electromagnetic spectrum by designating the frequency OR wavelength range of electromagnetic radiation because of wonderfully simple equation: c = f * λ. The speed of light (c), a constant, is equal to the product of frequency (f) and wavelength (λ). Once a wavelength is designated we automatically know the radiation’s frequency, as well as several other characteristics, namely photon energy. We can also classify regions of the electromagnetic spectrum by the interaction of electromagnetic radiation with matter. For example, microwave radiation interacts with matter by causing molecular rotation and vibration (heating up a cup of tea) while x-ray radiation possesses enough energy to ionize atoms (a medical CT scan). Ultraviolet (UV) radiation is a region of the electromagnetic spectrum that interacts with and excites atomic and molecular valence electrons, the electrons involved in chemical reactions. Indeed, the original literature on UV radiation, circa 1800-1850’s, referred to these light rays as chemical or oxidizing rays.
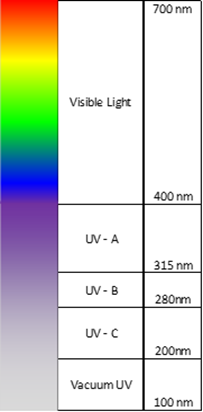
The figure above depicts the UV spectrum in its relationship to the recognizable visible spectrum. UV radiation is defined as radiation with wavelengths less than 400 nm and greater than 100 nm.Just as the visible spectrum is further divided into specific regions, known as ‘color’, so is the UV spectrum. Understanding these segments will later assist with photoinitiator selection and processing capabilities.
- The UV-A region extends just beyond our visible horizon and recent trends in photoinitiator chemistry employ this region more and more. This low frequency region has the highest depth of penetration into materials and the photons in this region as the safest to use (lowest energy).
- The UV-B region generally receives is classification due to its properties as a transition region. While most UV-A radiation does not have significantly damaging biological effects, the higher energy UV-B radiation does; you know this effect as sunburn.
- Many of the earliest UV-functional materials employed absorption in the UV-C region (e.g., benzophenone-type initiators). Wavelengths in this region are often referred to as “germicidal” wavelengths due to disruption of DNA processes at the cellular level. A further distinction of the UV-C region is that these wavelengths are almost completely absorbed the ozone layer while UV-B radiation is only partially absorbed (thank you ozone layer!).
- Vacuum UV is generally not employed by UV-curable resins but this region is extensively used in the photolithography industry.
While we are going to ignore photoinitiator reaction mechanisms for this post, I want to provide two examples of photoinitiator interaction with the UV spectrum. As mentioned earlier, a photoinitiator can absorb UV radiation, creating an excited electron state that induces a chemical reaction. However, each photoinitiator has a unique UV absorption fingerprint that will determine which wavelengths of radiation will create excited electron states and those that will not. In the plots below [1], the absorption fingerprint of two photoinitiators, benzophenone and triarylsulfonium hexafluorophosphate salt (THS), are shown with the photon wavelength on the x-axis and the absorption yield on the y-axis (0 implies no absorption and 1 implies complete absorption). Absorption can be concentration dependent but we will ignore the specific concentration values, listed in the plot legend as percentages, for this discussion and focus on the lowest concentration fingerprint, the green curves.
Benzophenone is nearly transparent to the UV-A and UV-B region and only in the highest frequencies of the UV-B region (~290 nm) does UV radiation begin to excite benzophenone valence electrons. The strongest absorption efficiencies do not occur until well into the UV-C with a peak absorption wavelength of 254 nm. Conversely, the THS molecule’s absorption spectrum begins to show strong absorption efficiencies in the middle UV-A region starting around ~340 nm. As you might expect, the differing absorption spectra of these two photoinitiators will have different requirements in manufacturing.
Benzophenone and benzophenone-type photoinitiators are among the shortest wavelength (highest frequency) absorbing photoinitiators for UV curable resins as oxygen begins to absorb electromagnetic radiation below 220 nm. THS only represents a “middle-ground” with respect to absorption in the UV-A region, several widely used photoinitiators have absorption spectra in the 360-380 nm range. There are also photoinitiator molecules with absorption fingerprints that extend into the visible region (> 400 nm) but we will save these molecules for another discussion.
[1] Sigma Aldrich Photoinitiators


can you please let know which process or instrument is needed for obtaining absorption finger print of Photoinitiator.
This is a nice set of articles. Very helpful. Thank you Jeff Gotro!
Btw, I am also a great fan of your old papers with Graessley!
Thank you for your kind words. I do have a small fan club of my work on the rheology of polyisoprenes and hydrogenated PI (nice analogs to EP copolymers).