We are back after a short hiatus over the holidays and now will do a series of posts on thermosets or thermosetting polymers.
 Thermosets as a class of polymeric materials don’t get a lot of play per se. When people think of polymers or plastics they typically identify the common plastics like polyethylene, polystyrene, and the ubiquitous polyethylene terephthalate (water bottles). But a major class of polymers used for adhesives, composites, laminates, coatings, encapsulants are made from thermosetting polymers.
Thermosets as a class of polymeric materials don’t get a lot of play per se. When people think of polymers or plastics they typically identify the common plastics like polyethylene, polystyrene, and the ubiquitous polyethylene terephthalate (water bottles). But a major class of polymers used for adhesives, composites, laminates, coatings, encapsulants are made from thermosetting polymers.
The Boeing Dreamliner fuselage is made using polymer composites. It has a highly engineered thermoset polymer matrix with very high performance fiber reinforcements to give it improved strength to weight.
The interesting feature of thermosets is that they undergo a chemical reaction during the curing or use. If you go to Home Depot or Lowe’s and grab some five minute epoxy, when you glue your parts together you have five minutes to make the bond. Well, what happens after five minutes? Actually five minutes is the gel time and after the gel time, it is difficult to flow and continue to process thermosets.
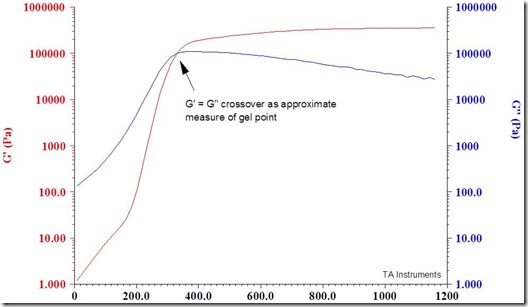
Well how do you know that five minutes is the gel time? Being thermoset guys, we put some five minute epoxy in a rheometer and measured the gel time. In the figure below, the dynamic moduli G’ (storage modulus, or elastic component) and G’’ (the loss modulus or viscous component) are plotted as a function of time during isothermal curing at room temperature (like you would use the glue at home!). The gel time is approximated by the crossover of G’ and G’’ (the dynamic moduli) which occurs right about 5 minutes.
Time (seconds)
The crossover occurs at approximately 300 seconds or 5 minutes. The gel point is a key feature of thermosetting systems. We will go into much more details about gelation and curing in future posts.
Another key aspect of thermosets is that they start as small molecules (monomers and oligomers) allowing easy dispensing or processing at low viscosity. During processing typically the temperature is increased causing the crosslinking reaction to start (via thermal initiators that react with the thermosets) leading to an increase in molecular weight (polymer chain length), but in contrast to thermoplastics, thermosets form links between the growing polymer chains which is termed crosslinking. The crosslinks are an important feature that gives thermosets high temperature stability and many of the properties associated with high modulus polymers.
Come back each week as we explore the interesting technology and applications of thermosetting polymers.

Leave a Reply