Thermosets (or thermosetting polymers) are an important class of polymeric materials. The major use of thermosets is in applications where high temperature stability is required. How do thermosets get this high temperature resistance? One word: crosslinks. Thermoplastics are high molecular weight linear (or sometimes branched) polymers. They derive their useful properties from the fact that the polymer molecules are very long chains, like a huge pearl necklace. One way to think of an amorphous polymer melt like polystryene is a bowl full of cooked spaghetti. This analogy doesn’t work so well with semi-crystalline polymers like polyethylene or polypropylene, but that’s another story.
Let’s get back to thermosets. The bowling ball above is made from a thermosetting polymer. If you put a polystryene cup (like a foam cup or the brittle clear injection molded cups) and a bowling ball in an oven and raise the temperature to about 150C, what would you expect to see after a while in the oven? Well, when you open the oven you would see a bowling ball and a puddle of liquid polymer in the bottom of the oven. The thermoplastic polymer would melt (or go through the glass transition temperature) and flow into a puddle. The bowling ball made of thermoset polymer might soften, but it will retain its shape due to the crosslinks formed during polymerization. Let’s take a closer look at thermoplastics:
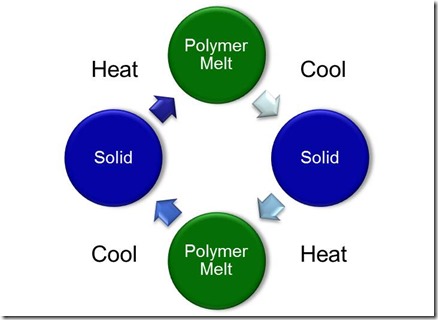
As we said before, thermoplastics are typically very high molecular weight linear or branched polymer chains and are solids are room temperature. If you apply heat such as melting in an extruder or injection molding machine, the polymer can soften, flow into the mold and be cooled back to a solid form in the shape desired. You can heat and cool thermoplastics several times. This is why you can recycle those PET (polyethylene terephthalate) water bottles.
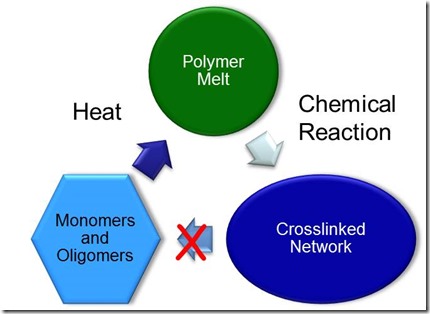
Thermosets on the other hand, start out as small molecules called monomers and oligomers (several monomers long) and then due to chemical reactions form crosslinks between the growing chains. These crosslinks then form a big network of tightly linked polymer chains. Since the chains are formed via a chemical reaction that links most of the long chains together, when you heat a thermoset, the part retains is shape. Let’s take a look at the schematic below:
Think about the 5 minute epoxy we discussed in the last post. It starts out as small molecules (epoxy, curing agents, diluents, fillers, etc.) and after you mix the two parts, a chemical reaction occurs and it becomes a very strong adhesive. Most adhesives are thermosets which allows them to be applied and then cured to glue your parts together. Circuit boards, composites (like the Boeing Dreamliner fuselage), car parts, boat hulls, and many more durable and heat resistant materials are made using thermosets.
in the next few posts we will dive into some of the important chemistries of thermosetting polymers.


Nice and informative article.
A good description about thermoplastic polymer create a sound knowledge Thanks for posting such an helpful article.
Thanks for this great article.
I have a question about aircraft polymer sealant PR-1436-G. Do you know what does the letter G stand for? And if this sealant is a type of thermoset?
B.Rs
Meysam