There are many emerging approaches to chemically recycling thermoset resins. This post will present an overview of the current approaches to address the end-of-life considerations in thermoset materials.
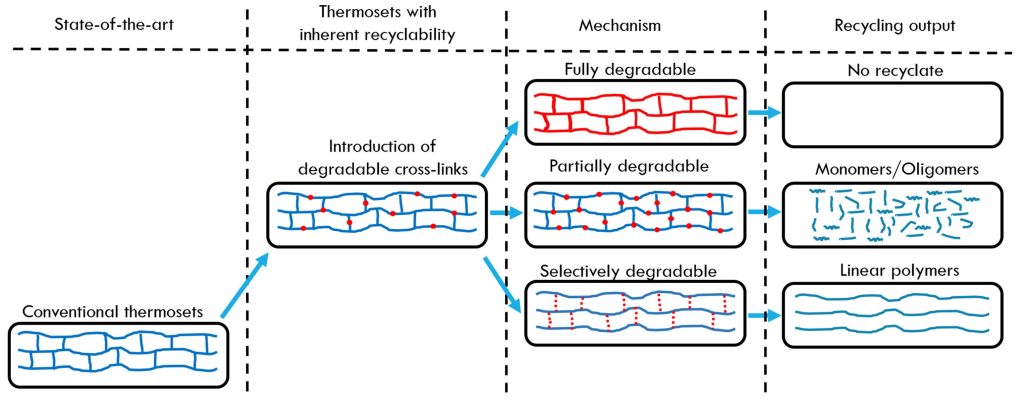
Figure 1 shows a schematic of the various approaches used to address the thermoset recycling challenge.
Figure 1. High level chemical recycling pathways [1]
The upper portion of the flow chart depicts the approach of using degradable linkages or incorporating degradable moieties into the thermoset network. The lower portion of the flowchart depicts the utilization of dynamic covalent networks or covalent adaptive networks (CANs) to achieve recyclability or reuse.
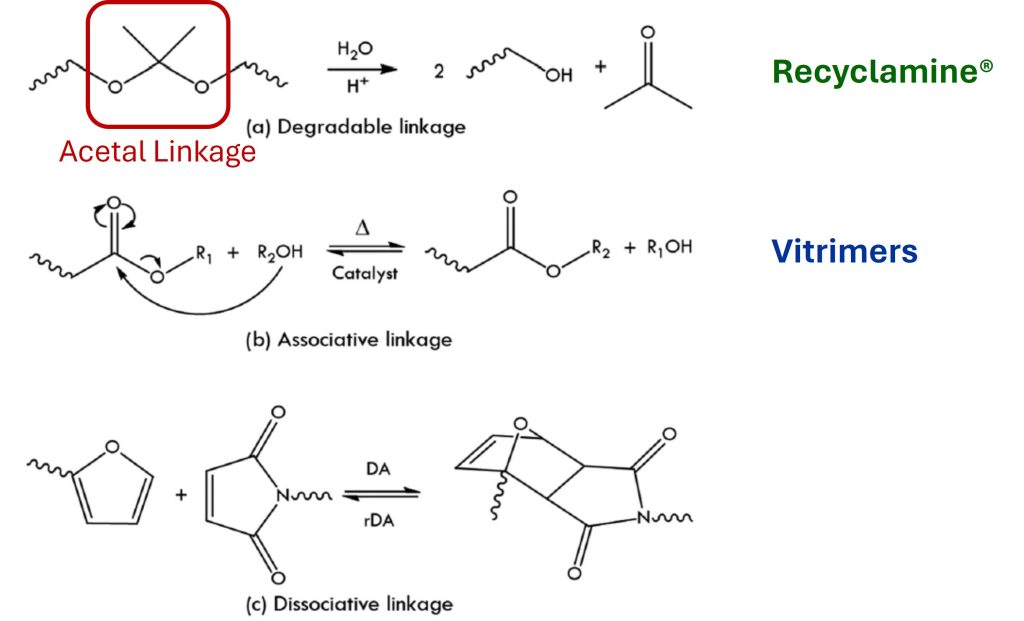
The chemical approaches used in the introduction of degradable crosslinks involves incorporating a cleavable linkage in the crosslinked polymer which is partially or selectively degradable. Using selectively degradable linkages (such as acetals) is the approach used in the Recyclamine® technology by Aditya Birla [2] as shown in Figure 2.
Figure 2. Molecular mechanisms for recycling thermosets [1]
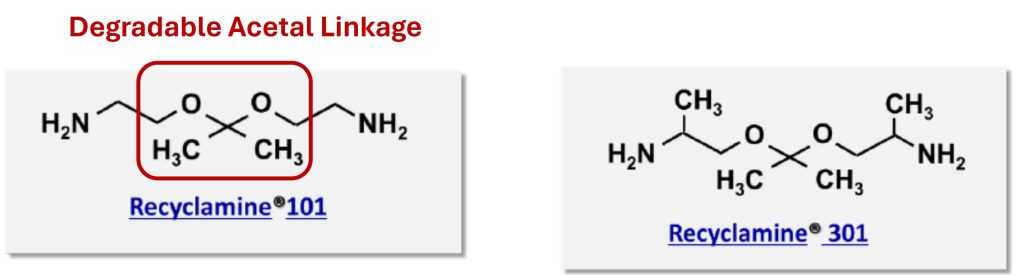
Recyclamine® 101 and Recyclamine® 301 are examples of selectively cleavable epoxy hardeners [2] as shown in Figure 3. The chemically cleavable moiety potentially allows for reprocessing, repairing, or recycling of crosslinked networks.
Figure 3. Recyclamine® examples from Aditya Birla [2]
The details of the Recyclamine® chemistry, material properties and processing will be covered in a subsequent post.
Vitrimers use reversible associative adaptive networks to allow for remolding and re-use as shown in Figure 2 and will be discussed in detail in the following section.
Covalent Adaptive Networks or Vitrimers
Vitrimers were first discovered by Professor Ludwik Leibler and his group at the Ecole Superieure de Physique et Chimie Industrielles, in Paris, France. The first paper describing vitrimers was published in SCIENCE in November 2011 [3]. In the paper, his group polymerized epoxy (DGEBA) with a mixture of dicarboxylic and tricarboxylic acids. The mixture was fully cured using known methods for epoxy curing.
One of the defining features of a vitrimer is the presence of what is now termed a covalent adaptive network abbreviated CAN. Other terms for this type of chemical structure are:
- dynamic covalent network
- reversible covalent bond containing polymers (RCBP)
- vitrimers
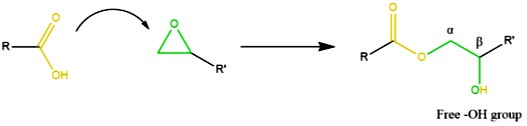
In Leibler’s pioneering work, the cured network contained a covalent adaptive network (CAN) as shown above. The key feature of the CAN is the presence of the ester linkage along with a nucleophile. In this case, the hydroxyl group (free -OH group) is electron rich due to the two lone electron pairs on the oxygen (hence the term nucleophile). The carbon in the carbonyl group (ester linkage) is electron deficient and thus is the site of the transesterification reaction in this type of dynamic covalent network. The transesterification reaction led to a topological rearrangement that led to an unusual change in the viscoelastic response at elevated temperatures. At elevated temperatures, the viscosity behaved like vitreous silica, hence Liebler coined the term “vitrimer.”
Part Three in this series will describe the details of the chemistry of vitrimers and Part Four in this series will cover the details of the viscoelastic properties of vitrimers.
References
- POLYMER REVIEWS, VOL. 60, NO. 2, 359-388, (2020) https://doi.org/10.1080/15583724.2019.1673406
- Dubey et. al., Recyclamine® – Novel Amine Building Blocks for a Sustainable World, SAMPE neXus Proceedings. Virtual Event, June 29 – July 1, (2021). Society for the Advancement of Material and Process Engineering – North America.
- Liebler, et. al., www.sciencemag.org SCIENCE vol 334 18 November (2011)

Leave a Reply