The last post introduced vitrimers. This post will provide more detailed information regarding the chemistry of the covalent adaptive networks in vitrimers.
In Leibler’s pioneering work, the cured network contained a covalent adaptive network (CAN).
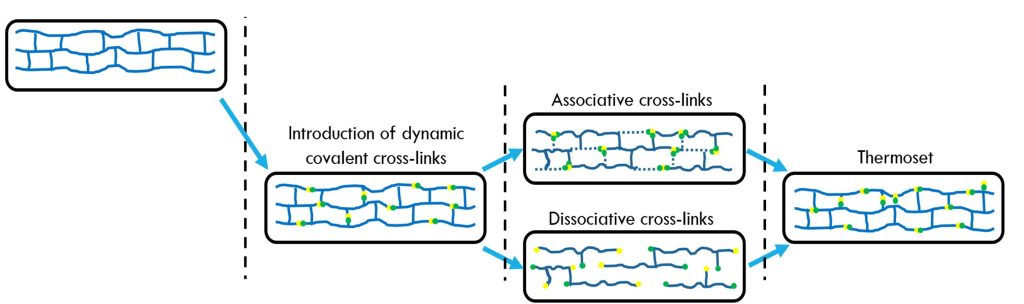
Figure 1. Schematic approach to vitrimers [1]
In Figure 1, there are two types of covalent adaptive networks (CANs):
- associative CANs
- dissociative CANs.
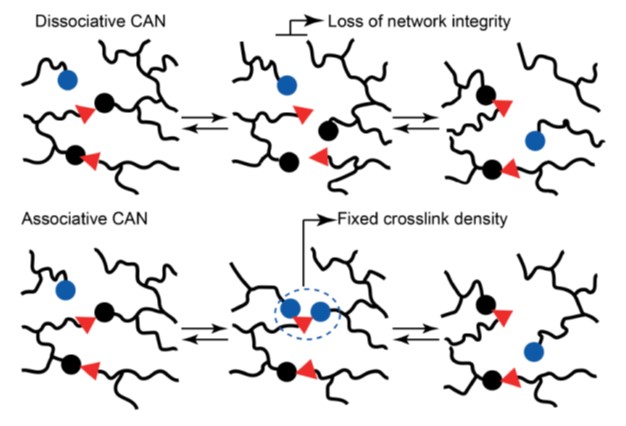
The two types of covalent adaptive networks are shown schematically in Figure 2.
Figure 2. Schematic of the two types of covalent adaptive networks [2]
In the case of dissociative CANs, there is a loss of network integrity, i.e., the covalent bonds in the network chains are broken. On the other hand, in the case of associative CANs, the network integrity is maintained, i.e., the crosslink density remains constant since the covalent bonds topologically rearrange via the adaptive covalent bonds but are not broken. This is the key new concept in vitrimer processing.
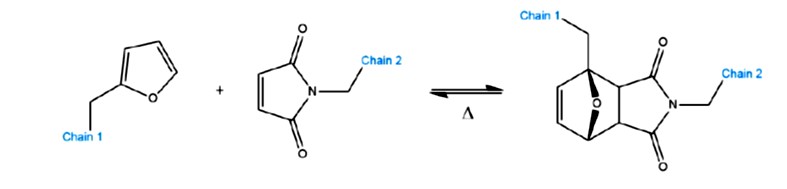
An example of a dissociative covalent adaptive network is the Diels Alder and retro (or reversible) Diels Alder cycloaddition as shown in Figure 3.
Figure 3. Diels Alder dissociative covalent adaptive network [3]
In this type of dissociative CAN, the Diels Alder reaction (either forward or retro) is driven by temperature. During curing at elevated temperatures, the reaction in Figure 3 proceeds to the right yielding a cyclic structure. After curing, the network is stable at lower temperatures (i.e. much lower than the cure temperature). At higher temperatures, the reverse (or retro) Diels Alder reaction pathway drives the reaction towards the left side in Figure 3. When the retro Diels Alder reaction occurs, there is a rapid drop in the viscosity due to the transition to smaller molecules. In this case, the crosslink density is dramatically decreased. As the temperature is lowered, the Diels Alder cycloaddition reverts (mostly) to the cyclic structure on the right in Figure 3.
Vitrimers make use of associative CANs where the network integrity is maintained, i.e. the crosslink density remains essentially constant since the covalent bonds topologically rearrange but are not broken.
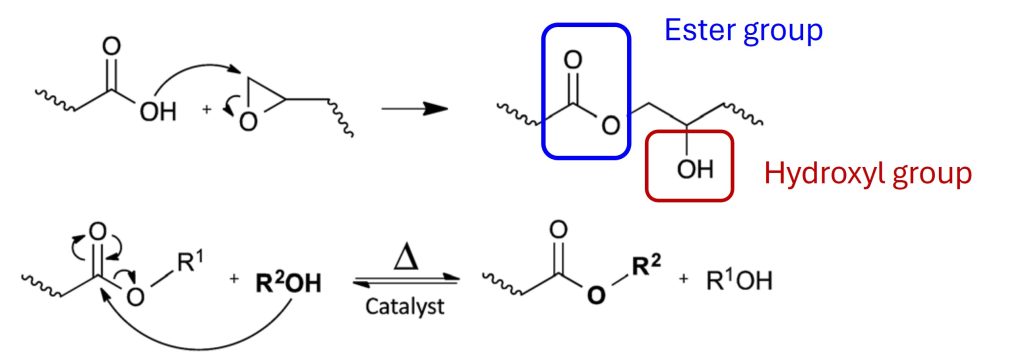
Figure 4. Vitrimers contain associative covalent adaptive networks [3]
In Figure 4, the epoxy reaction with carboxylic acid is shown. Recall this was the chemistry used by the Leibler group whereby they synthesized epoxy networks by classical epoxy chemistry; the reaction of diglycidyl ether of bisphenol A (DGEBA) cured with a mixture of fatty dicarboxylic and tricarboxylic acids [4]. The epoxy/COOH stoichiometry was 1:1 to have both -OH and ester groups in the final network. At room temperature, the crosslinked network had the properties of an elastomer. In Figure 4 there are two chemical linkages to note; the first is the ester linkage and the second is the hydroxyl group. In this case, the vitrimer behavior is caused by the transesterification reaction via the -OH nucleophile shown in the second line in Figure 4. The transesterification reaction in Figure 4 does not occur (or occurs very slowly) in the absence of a catalyst. Leibler et. al. [4] showed the addition of a metal catalyst such as zinc acetate (Zn(Ac)2) at 5 mole% was required to achieve transesterification.
In the same paper, the Leibler group demonstrated the concept of associative CANs can be used to synthesize networks that are hard at room temperature and be malleable and moldable at elevated temperatures. Epoxy resins cured with anhydrides yield networks containing -OH and ester linkages which could undergo transesterification reactions (associative CANs) at elevated temperatures. Networks were synthesized using DGEBA and glutaric anhydride with the epoxy/acyl stoichiometric ratio at 1:1 in the presence of 5 or 10 mole% zinc acetyl acetonoate (Zn(AcAc)2).
Common associative covalent adaptive networks in vitrimers can be:
- Transesterification (hydroxyl groups)
- Tranamination (amine groups)
- Disulfide exchange (sulfur groups)
- Other chemistries, see reference 3 for more examples.
The next post will cover the viscoelastic response associated with vitrimers.
References
- POLYMER REVIEWS, VOL. 60, NO. 2, 359-388, (2020) https://doi.org/10.1080/15583724.2019.1673406
- Denissen,W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Sci., 7, 30–38 (2016)
- Alabiso, W., and Schlogl, S., Polymers, 12, 1660, (2020) https://dx.doi.org/10.3390/polym12081660
- Liebler, et. al., www.sciencemag.org SCIENCE vol 334 18 November (2011)

Leave a Reply