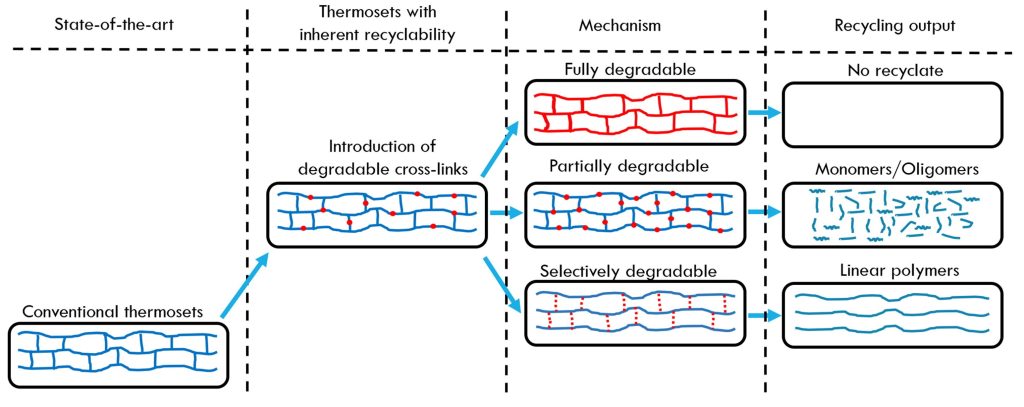
The previous five posts discussed the end-of-life considerations using thermosets with covalently adaptive networks termed vitrimers. The next two posts will cover the chemical degradation approach to thermoset recycling. As shown schematically in Figure 1, there are multiple approaches to degrade fully cured thermoset networks.
Figure 1. Schematic of chemical degradation methods for recycling [1]
The fully degradable route is focused on bio-based thermosets and will not be covered here. Another chemical route is to partially degrade the thermoset into monomers and oligomers. The most common chemical route is to selectively degrade specific linkages added to cure the network and will be the focus of this post.
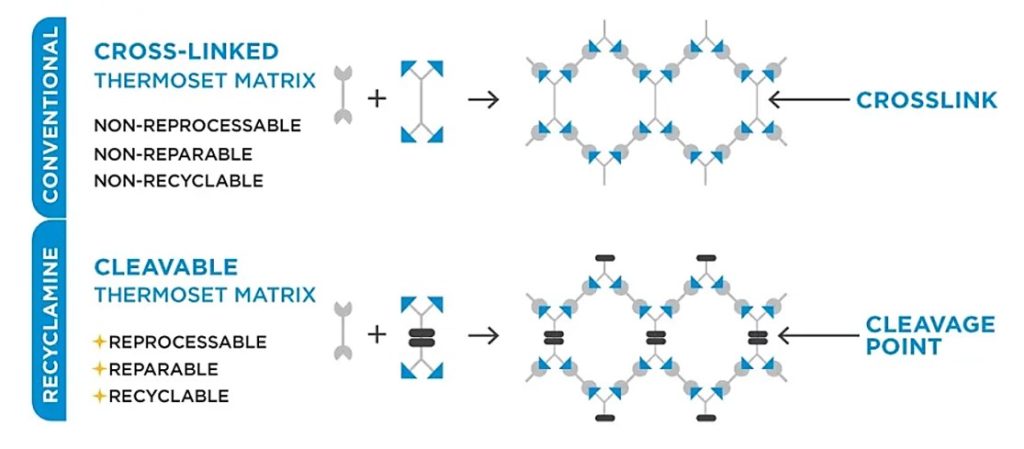
Recyclamine® hardeners were first developed and patented by Connora Technologies [2] and are now commercially available from Aditya Birla (see www.recyclamine.com/recyclamine-technology). The concept was to enable the cleavage of a specific linkage in the epoxy network that would enable post-processing. Figure 2 shows a schematic of a traditional epoxy network compared with a network cured with a Recyclamine® hardener.
Figure 2. Schematic of chemical degradation method that incorporates a chemical cleavage linkage [3]
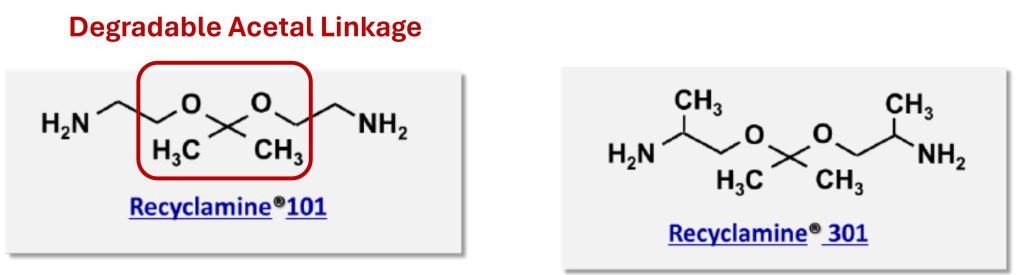
Conventional thermoset materials are challenging to recycle or reuse. The inclusion of a chemically cleavable moiety potentially would allow for reprocessing, repairing, or recycling of crosslinked networks. Recyclamine® hardeners are commercially available in multiple different chemistries [4]. Some of the commonly used hardeners are Recyclamine® 101 and Recyclamine® 301 as shown in Figure 3.
Figure 3. Recyclamine® hardeners with a chemically cleavable linkage [2-4]
These Recyclamine® hardeners contain an acetal linkage as highlighted on the left side in Figure 3. The ether linkages (-C-O-C-) can be cleaved when the crosslinked resin is immersed in acidic solution at elevated temperatures (80-90°C) for several hours. Another class of Recyclamine® hardeners contains a silicon-oxygen linkage as shown in Figure 4. There are 7 classes of Recyclamine® hardeners denoted by 100, 300, 400, 500. These all contain some form of ether linkages (-C-O-C-) with the “01” in the nomenclature indicating the acetal functionality (see 101 and 301 in Figure 3). The paper by Dubey et. al., [4] describes the complete line-up of Recyclamine® hardeners
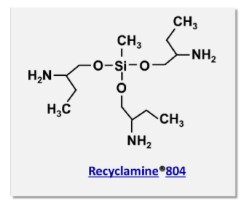
In addition, the 700, 800, and 900 series hardeners contain multifunctional silicon containing linkages (-C-O-Si-O-C-) as shown in Figure 4, where the Recyclamine® 804 hardener is shown.
Figure 4. The chemical structure of Recyclamine® 804 [4].
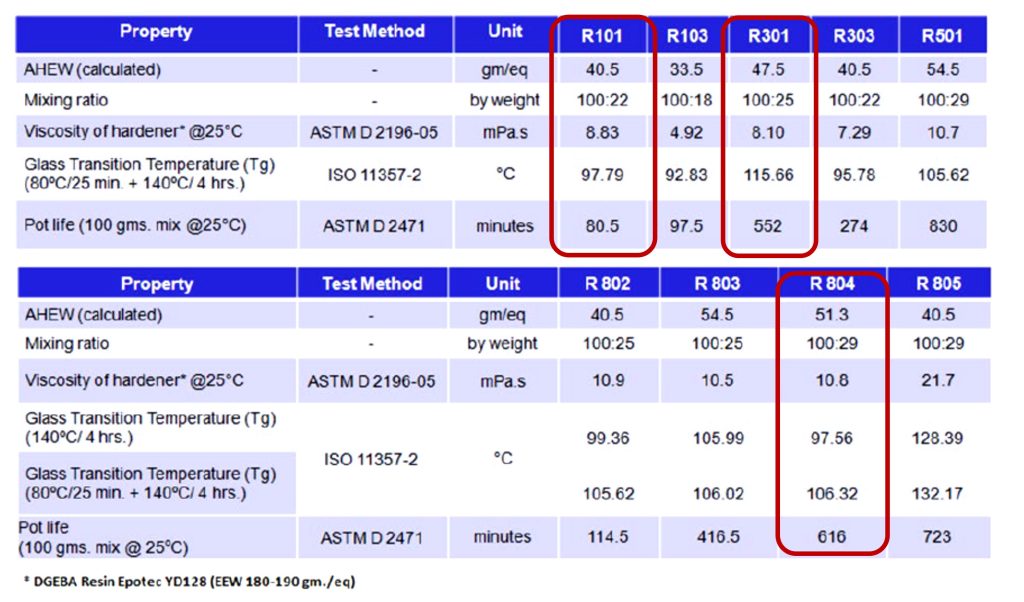
In the SAMPE paper by Dubey et. al., data is provided for model epoxy formulations using diglycidyl ether of bisphenol A (Epotec® YD128 with EEW of 180-190 g/eq) and various Recyclamine® hardeners. The amine hydrogen equivalent weights and the mixing ratios for the various formulations are shown in Table 1.
TABLE 1.
The Recyclamine® hardeners are primary amines i.e., with the -NH2 group present in the hardeners and are typically very reactive. As shown in Table 1, formulated epoxies have good pot life at 25°C so may be used in a variety of composite manufacturing processes such as wet lay-up, resin infusion molding, vacuum assisted resin transfer molding (VARTM) and other composite processes [4]. Recyclamine® hardeners have low viscosity and when formulated with a typical DGEBA epoxy resin achieved mid-range glass transition temperatures when cured at 140°C as shown in Table 1.
The next post will cover the chemical degradation process to recover the high value added carbon fiber or glass fibers.
References
- POLYMER REVIEWS, VOL. 60, NO. 2, 359-388, (2020) https://doi.org/10.1080/15583724.2019.1673406
- Patents covering Recyclamine® hardener technology assigned to Connora Technologies; CA2819759 WPO Publication, EP264610B, US9862797, US10214479, US20190016667A1
- recyclamine.com/recyclamine-technology
- Dubey et. al., Recyclamine® – Novel Amine Building Blocks for a Sustainable World, SAMPE neXus Proceedings. Virtual Event, June 29 – July 1, (2021). Society for the Advancement of Material and Process Engineering – North America.

PETG has a wide application in bottles and signboards. If we can recycle these objects are made from PETG, there will be a great benefit for environmental protection.