The previous post described the work of Liebler [1] who showed both epoxy carboxylic acid networks and epoxy anhydride networks contained covalent adaptive networks that underwent transesterification reactions at elevated temperatures (i.e. vitrimers).
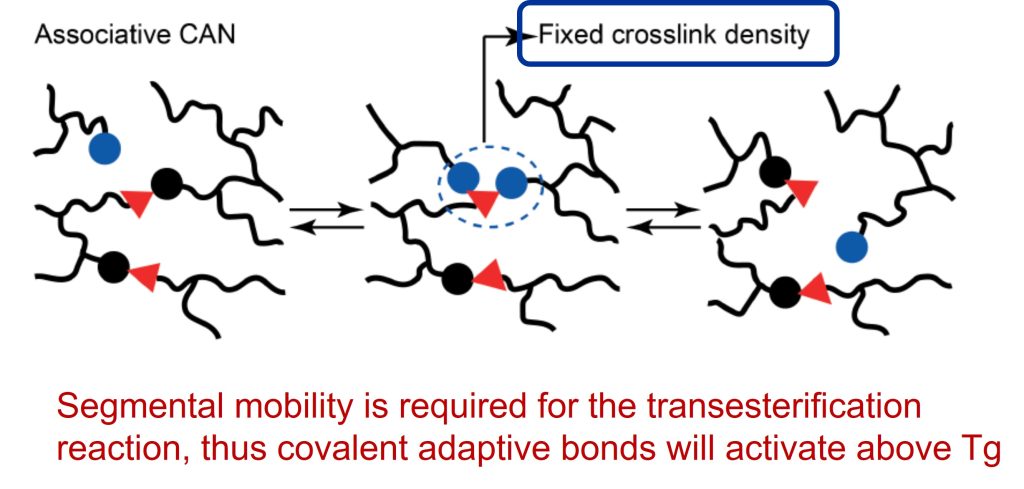
The unique discovery in both epoxy systems was the viscoelastic behavior at elevated temperatures. Vitrimers are covalently bonded crosslinked networks. These networks can change their viscoelastic properties via a chemical exchange reaction (i.e. associative covalent adaptive network) that are triggered at elevated temperatures. The viscosity is controlled by the kinetics of the exchange reactions, typically decreasing via an Arrhenius rate equation. The temperature where the viscosity transition occurs is termed Tv or the topology freezing transition temperature. Leibler chose the point where the viscosity reached 1012 Pa-s [1]. It is important to recall that vitrimers are permanently crosslinked networks at all temperatures (lower than the degradation temperature) and do not dissolve in chemically inert solvents.
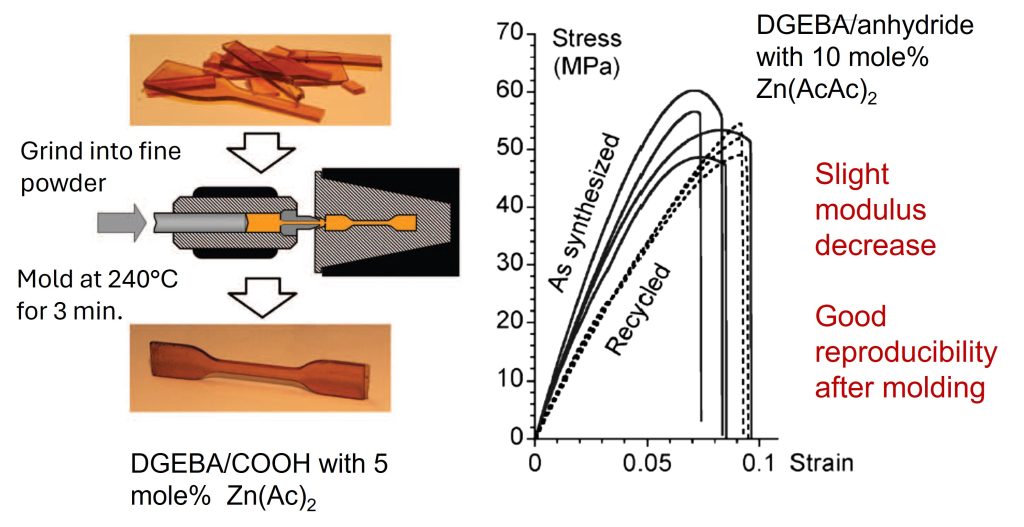
In the case of the epoxy/carboxylic acid networks, broken or ground powders could be molded despite being permanently crosslinked and well beyond the gel point [1] as shown in Figure 1.
Figure 1. Crosslinked epoxy sample reprocessed using injection molding [1].
In a similar manner, the fully cured epoxy anhydride network described above had properties typical of a hard epoxy resin with a Tg in the range of 80°C and a modulus of 1.8 GPa at room temperature. In contrast to ordinary epoxy/anhydride cured networks, the catalyzed epoxy/anhydride system with 10 mole% Zn(AcAc)2 was able to flow at 200°C as a result of the transesterification reactions and the resulting topological rearrangements in the network with associative CANs.
In Figure 1, the stress strain curve is presented for the as-synthesized (or fully cured) 10 mole% Zn(AcAc)2 catalyzed DBEBA/anhydride resin and exhibits characteristics of a hard, fully cured epoxy network (high Young’s modulus and low elongation at break). The dashed curve in Figure 1 (labeled recycled) was obtained by grinding the fully cured sample into a fine powder and compression molded at 240°C for three minutes. The recycled sample had a slightly lower modulus but similar elongation at break indicating the recycled vitrimer network was still a highly crosslinked material.
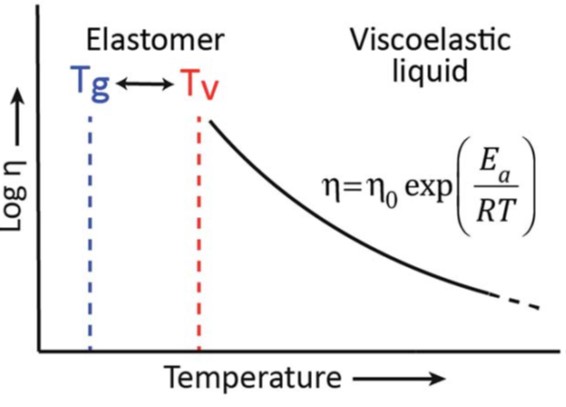
For an elastomer, the Tg is lower than room temperature yielding a rubbery-like network. Since this type of network is crosslinked, then there is some limited segmental mobility, but not enough to allow the exchange reactions (i.e. transesterification in the epoxy/COOH example). At a higher temperature, the kinetics of the exchange reactions becomes rapid enough to cause the viscosity to decrease. The key finding from Liebler’s work is that the viscosity decreases more gradually compared to a typical polymer undergoing a glass transition temperature on heating. The viscosity decrease follows an Arrhenius relationship above the temperature where the exchange reactions start. Leibler called this point the “topology freezing transition temperature” or Tv and assigned a viscosity of 1012 Pa-s. The relationship between Tg and Tv for an elastomer (such as the epoxy/COOH system) is shown schematically in Figure 2.
Figure 2. Tg and Tv for an elastomeric crosslinked network [2]. Note that for an elastomer, room temperature would be between Tg and Tv.
In the schematic in Figure 2, an elastomer will be flexible at room temperature (since Tg < RT). During an increasing heating profile starting at sub-ambient, the network will be in the glassy state below Tg. As the temperature increases to above Tg, an elastomeric network will exhibit rubbery behavior, but will not flow due to the crosslinked network structure. In this elastomeric network the segmental mobility may not be enough to allow for associative covalent adaptive networks to rearrange. At some temperature, the exchange reactions (i.e., transesterification) has enough thermal energy to initiate the changes in network topology. Leibler termed this temperature Tv or the topological freezing transition temperature. In the increasing heating example, the Tv is the topological softening temperature, since above this temperature the kinetics of the covalent adaptive network allow topological rearrangement of the network chains.
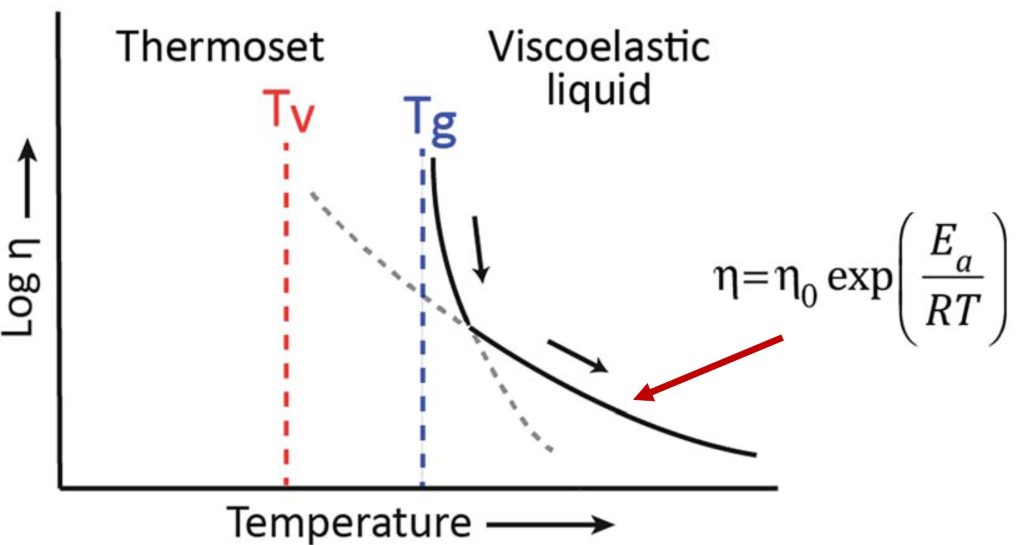
The situation is different for a highly crosslinked hard thermoset network (such as the epoxy/anhydride network described above). In this case, when the temperature is increased from sub-ambient, the highly crosslinked network is in the glassy phase and exhibits a high Tg, high modulus, and there is very limited segmental mobility in the glassy phase. The limited segmental mobility arises for two reasons; the temperature is low; thus the chains don’t have enough thermal energy to move past each other and more importantly, the network has high crosslink density which significantly impedes segmental mobility. At the glass transition temperature, the network transitions from a glassy polymer network to a rubbery network (i.e. devitrifies). Depending on the crosslink density, the mobility of the polymer chains above Tg is still relatively restricted.
For a network that contains covalent adaptive networks, as the temperature increases, the exchange reactions can initiate. If the network is still below the Tg, the segmental mobility in the glassy state will likely suppress any topological rearrangements even though they may be kinetically activated. Upon further heating, the network will go through Tg resulting in a large drop in viscosity and increase in segmental mobility. This is shown schematically in Figure 3.
Figure 3. Tg and Tv for a hard crosslinked network [2]. Note that for a rigid thermoset, room temperature would be below Tv and Tg.
Recall that networks with CANs are fully crosslinked during curing so they will exhibit the classical viscosity/modulus decrease at Tg, as shown in Figure 3. Above Tg, the increased temperature and segmental mobility will allow for the activation of the associative covalent networks (i.e., transesterification).
The viscoelasticity of the CAN networks will then follow an Arrhenius type relationship as shown in Figure 3.
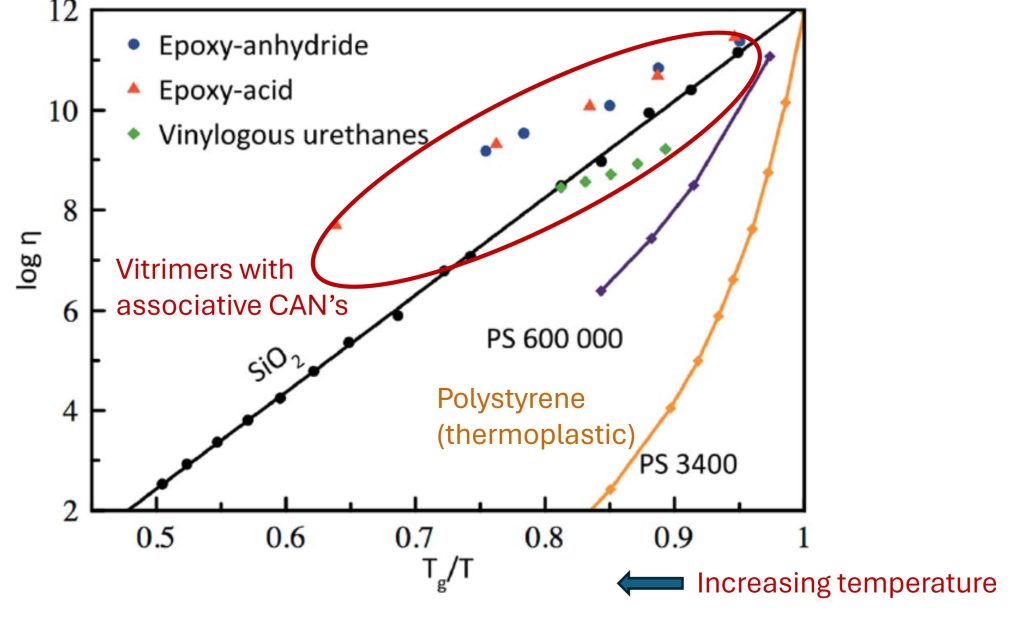
The rate of viscosity change at the glass transition temperature (“fragility”) indicates the breadth of the glass transition region [3]. Materials like vitreous silica have very broad Arrhenius type behavior in the glass transition region and are termed “strong” glass formers. Polymers are more “fragile” as they show a very pronounced drop in viscosity at Tg on heating.
Figure 4. Viscosity inverse temperature plot for various materials [2]
Figure 10 called a “fragility plot” [3] where the viscosity is plotted as a function of normalized inverse temperature (temperature is decreasing moving towards the left in Figure 10), where three types of resin systems containing associative covalent adaptive networks are plotted. The epoxy-acid and the epoxy anhydride are the adaptive networks described above and discovered by Liebler et. al. [1]. For reference, polystyrene in various molecular weights is plotted for reference. Polystyrene is an amorphous glassy thermoplastic polymer that exhibits a distinct Tg (~105°C). Note the viscosity decreases rapidly above Tg whereas the network with CANs has a much slower decrease as the temperature increases.
In Figure 4, the viscosity decrease for vitreous silica (SiO2) is plotted for reference. The viscosity temperature relationship for the three CANs is plotted and noted in the red oval. In contrast to the polystyrene viscosity response, the three vitrimer networks with associative CANs follow closely the Arrhenius type viscosity profile typical of silica glasses. It should be noted that the transition from a liquid to a glassy solid during cooling is termed vitrification. Traditional thermosets “devitrify” or transition from the glassy state to the rubbery or liquid state at the glass transition temperature. The discovery of the viscoelastic response for the exchange reactions in these types of covalent adaptive networks was like that of vitreous silica led Leibler to call these materials “vitrimers.”
Since Leibler’ s original work, many researchers have been working on vitrimers and there are some good review articles available [4-16].
A comprehensive review of thermosets based on reversible covalent bonds (vitrimers) may be found in the Handbook of Thermoset Plastics, Chapter 16, “Thermosets Based on Reversible Covalent Bonds (Vitrimers) [17].
Part Five in this series will focus on the use of vitrimers in thermoset applications.
References
- Liebler, et. al., www.sciencemag.org SCIENCE vol 334 18 November (2011)
- Denissen,W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Sci., 7, 30–38 (2016)
- Angell, C. A, Science 267,1924 (1995)
- POLYMER REVIEWS, VOL. 60, NO. 2, 359-388, (2020) https://doi.org/10.1080/15583724.2019.1673406
- Alabiso, W., and Schlogl, S., Polymers, 12, 1660, (2020) https://dx.doi.org/10.3390/polym12081660
- Leibler et. al., J. Am. Chem. Soc., 134, 7664−7667 (2012) https://dx.doi.org/10.1021/ja302894k
- Sokolov et. al., Phys. Chem. B2021, 125, 33, 9389–9401 Publication Date:July 29, 2021 https://dx.doi.org/10.1021/acs.jpcb.1c03511
- Schenk et. al., Adv., 3, 8012–8029, (2022)
- Ye, C. (2022). Design of sustainable polymer networks for advanced applications based on dynamic covalent bonds. [Thesis fully internal (DIV), University of Groningen]. University of Groningen. https://doi.org/10.33612/diss.215510652
- Zhou et al., Cell Reports Physical Science 3, 101036, September 21, 2022 https://doi.org/10.1016/j.xcrp.2022.101036
- Rahman et al., Cell Reports Physical Science 4, 101695, (2023) https://doi.org/10.1016/j.xcrp.2022.101036
- Zhang, J. al., Polymer, 194, p. 122392 92020) https://doi.org/10.1016/j.polymer.2020.122392
- Du Prez, et. al., Chem, Sci., 11, 4855 (2020), https://doi.org/10.1039/d0sc01069c
- Zhang, J. et. al., Macromolecules 51, 5577-5585 (2018) https://doi.org/10.1021/acs.macromol.8b01010
- Zhang, J. et. al., Macromolecules 51, 6789-6799 (2018) https://doi.org/10.1021/acs.macromol.8b01424
- Taynton, et. al., Materials, 2014, p. 1 https://doi.org/10.1002/adma.201400317
- Handbook of Thermoset Plastics, Chapter 16, “Thermosets Based on Reversible Covalent Bonds (Vitrimers), Fourth Edition, Edited by Hanna Dodiuk, ISBN:978-0-12-821632-3, Elsevier, (2022)

Leave a Reply