Guest Post by Dr. R. Bruce Prime
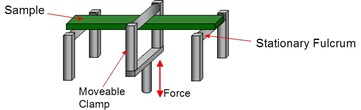 In this post we describe how DMA can contribute to the study of cure kinetics. We will concentrate on areas where DSC fails to yield useful kinetic data or where Tg is the preferred or even the only way to get a handle on the kinetics. One example is phenolic based thermosets described in the last post, where the endothermic mass loss interferes with the cure exotherm and renders it useless as a measure of conversion. The thermoset system described below that contains a high-boiling solvent is another. In both cases Tg can easily be measured by DMA, allowing time-temperature superposition (TTS) kinetic analysis by invoking the Tg – conversion relationship. In addition DMA can measure gelation which allows measurement of activation energy from gel time vs. temperature measurements. Recall from the May 12, 2014 post, that gelation is an isoconversional property. And finally we define the DMA Degree of Cure (DOC) and show how that can be used in conjunction with TTS to model cure behavior.
In this post we describe how DMA can contribute to the study of cure kinetics. We will concentrate on areas where DSC fails to yield useful kinetic data or where Tg is the preferred or even the only way to get a handle on the kinetics. One example is phenolic based thermosets described in the last post, where the endothermic mass loss interferes with the cure exotherm and renders it useless as a measure of conversion. The thermoset system described below that contains a high-boiling solvent is another. In both cases Tg can easily be measured by DMA, allowing time-temperature superposition (TTS) kinetic analysis by invoking the Tg – conversion relationship. In addition DMA can measure gelation which allows measurement of activation energy from gel time vs. temperature measurements. Recall from the May 12, 2014 post, that gelation is an isoconversional property. And finally we define the DMA Degree of Cure (DOC) and show how that can be used in conjunction with TTS to model cure behavior.
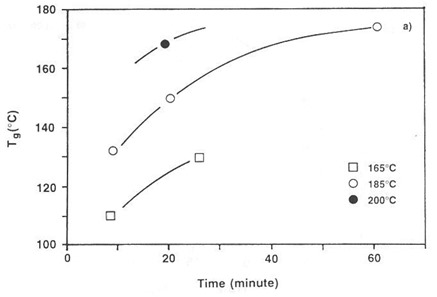
The figure below shows Tg vs. time at three temperatures for a thermosetting paste. The paste was used for screen printing conductive elements to repair printed circuit boards. Because the paste contained a high-boiling solvent it was not possible to measure a clean DSC exotherm, whereas Tg could easily be measured by DMA after partial cure.
Tg after isothermal cure in convection oven. Thermosetting paste on 100 mm thick wire mesh. From Prime, Polym. Eng. Sci. 32, 1276 (1992).
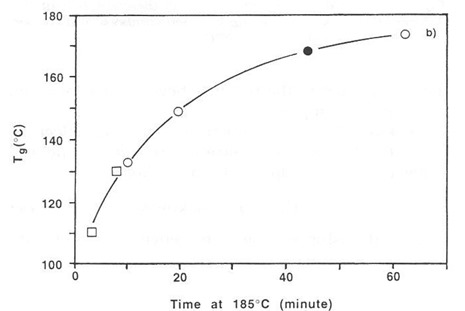
In spite of the lack of an independent measure of activation energy and only six data points it was still possible to construct a reasonable master curve, shown below. The reference temperature was selected as the middle temperature (185°C) and simultaneously the 200°C data was shifted to the right and the 165°C data to the left by varying the activation energy in an Excel spreadsheet until visually the best master curve was obtained. An activation energy of 22.5 ± 1 kcal/mole was measured in this way.
Master cure curve for thermosetting paste. From Prime, Polym. Eng. Sci. 32, 1276 (1992).
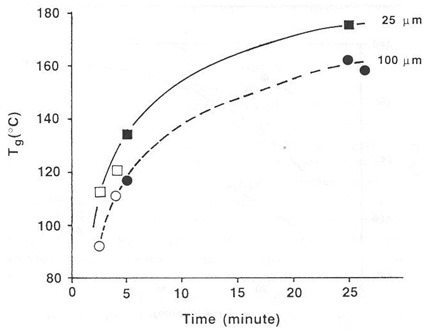
Additional experiments were performed using 25 mm thick wire mesh and by curing in an IR oven. From the figure below it was concluded that only time at temperature determined the extent of cure, and not the type of oven. However, sample thickness did play a role, which was attributed to slow evaporation of the high-boiling solvent.
Tg vs. time at 165°C for thermosetting paste on different thicknesses of wire mesh following oven cure (solid symbols) and IR cure (open symbols). From Prime, Polym. Eng. Sci. 32, 1276 (1992).
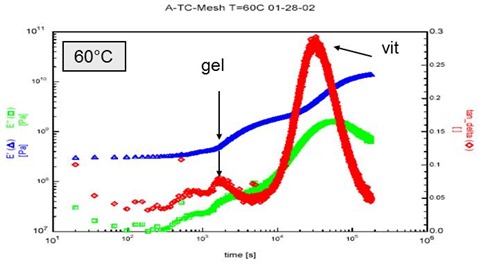
The figure below shows cure in the DMA for a water-borne coating. Gelation is observed from the onset of the first step increase in storage modulus and the small peak in tan d. Vitrification is observed later as the second step increase in storage modulus and a larger tan d peak.
For a given thermosetting system gelation will always occur at the same conversion, independent of temperature. Factoring this into Eq. 1 in Part 1 leads to the following
From Eq. 25 we observe that the time to gel is inversely proportional to the rate constant k. We substitute for k in Eq. 2, leading to
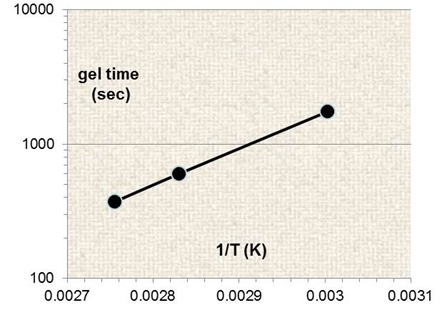
It follows that a plot of ln (tgel) vs. T-1 will have a slope of E/R. The figure below is such a plot for the water-borne coating at three temperatures. The slope is 6,230K and E = 6,230K ´ 1.99 cal K-1 mole-1 = 12.4 kcal mole-1.
Time to gel vs. T-1 for water-borne coating. From Eng, McGrath, Pilgrim, Prime, 30th NATAS Poster, 2002.
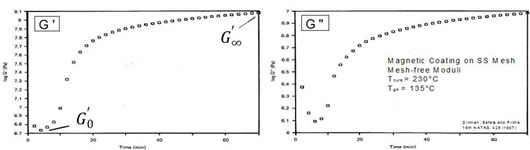
In the final example we define and illustrate the DMA degree of cure (DOC). This technique utilizes the relationship between the rubbery modulus and the crosslink density {Flory and Rehner, J. Chem. Phys. 11, 521 (1943)}. This method applies to cure between the gel point and the end of cure in the absence of vitrification, as illustrated in the figure below and defined by Eq. 27.
Isothermal cure of thermoset magnetic coating in N2. Tcure = 230°C and Tg¥ = 135°C, precluding vitrification during cure. From Dillman, Seferis and Prime, 16th Proced. NATAS Conf. 429 (1987).
where ![]() is the storage modulus at the gel point,
is the storage modulus at the gel point, ![]() is the rubbery storage modulus at full cure and
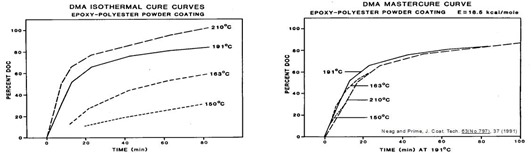
is the rubbery storage modulus at full cure and ![]() is the storage modulus along the cure path. The figure below illustrates application to the isothermal cure of a coating. Samples on wire mesh were cured in the DMA and the curves shifted to times a reference temperature to form a master cure curve. An activation energy of 18.5 kcal/mole was obtained from the shifting process.
is the storage modulus along the cure path. The figure below illustrates application to the isothermal cure of a coating. Samples on wire mesh were cured in the DMA and the curves shifted to times a reference temperature to form a master cure curve. An activation energy of 18.5 kcal/mole was obtained from the shifting process.
Epoxy-polyester powder coating. DMA DOC vs. time at four temperatures (left). Curves shifted to times at 191°C to form master cure curve (right). From Neag and Prime, J. Coat. Tech. 63(No. 797), 37 (1991).
A single heating rate DSC method overestimated the actual cure, whereas the DMA DOC master curves gave an accurate prediction of isothermal cure and a good correlation with impact resistance.

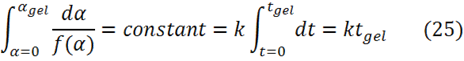
![clip_image002[7] clip_image002[7]](https://polymerinnovationblog.com/wp-content/uploads/2014/12/clip_image0027_thumb.png)
![clip_image002[9] clip_image002[9]](https://polymerinnovationblog.com/wp-content/uploads/2014/12/clip_image0029_thumb.png)
Hi,
I’m planning to use a DMA equipment to study the cure behavior of a waterbased coating. I know that I can use for example a composite bar as substrate and I have to cast my latex on it. But my question is, do I have to wait to form a dry film to perform the DMA test? Because it’s not totally clear for me that if I wait to film to dry how can I measure the gel point. I mean, once the film is dried how can I recover the information about the gel point using the DMA if the gel point was related with the film formation?
Thank you