Guest Post by Dr. R. Bruce Prime
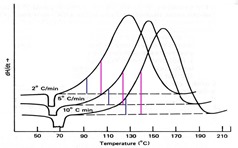 In previous posts we showed how to begin a kinetic study and one means to measure activation energy E. Here we show how DSC may be used to measure the parameters in the rate equations, a and da/dt, that can lead to elucidation of the entire rate equation, including another means to measure E. Because DSC gives a direct measure of both the conversion a from the area under the DSC exotherm and the rate of conversion da/dt from the y-axis (dH/dt or heat flow) it is the analytical tool most used to measure cure kinetics.
In previous posts we showed how to begin a kinetic study and one means to measure activation energy E. Here we show how DSC may be used to measure the parameters in the rate equations, a and da/dt, that can lead to elucidation of the entire rate equation, including another means to measure E. Because DSC gives a direct measure of both the conversion a from the area under the DSC exotherm and the rate of conversion da/dt from the y-axis (dH/dt or heat flow) it is the analytical tool most used to measure cure kinetics.
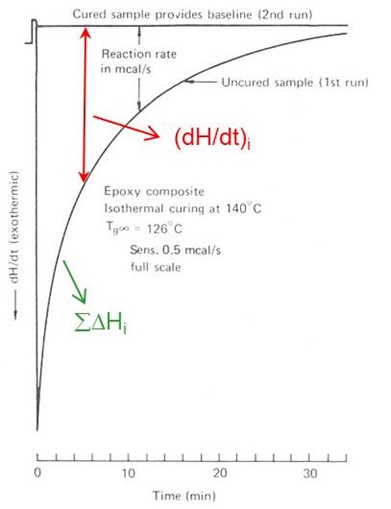
There are two isothermal DSC methods, referred to as Method 1 and Method 2. In Method 1 samples are cured by ramping the DSC quickly to Tcure and holding until the reaction is complete. The baseline may be drawn between the beginning and end of the reaction or from a 2nd run on the cured sample, as illustrated in the figure below.
Isothermal DSC of uncured thermoset heated from 50 to 140°C at 320°C/m.
From A. Gray, Perkin-Elmer TAAS 2 (1972).
In Isothermal Method 1 conversion a vs. time is measured from the cumulative area under the exotherm
where irefers to incremental measurements and DHrxn is measured from scanning experiments, e.g. at 5 or 10°C/min. The rate of conversion da/dt is measured from the DSC amplitude dH/dt
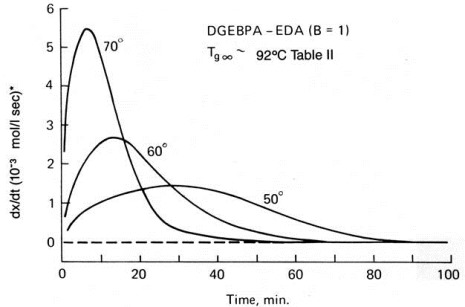
Notice that the maximum in the exotherm occurs at the start of the reaction which tells us that this is an nth order reaction (from Eq. 3 in Part 1 of this series the maximum rate occurs at t = 0). The maximum in the isothermal DSC curves below occur at t > 0, with the tmax increasing with increasing temperature. This behavior is due to the increasing concentration of the alcohol produced in the epoxy-amine reaction (Scheme 2 in Part 1) balanced by the consumption of the epoxide and amine reactants.
Isothermal DSC of epoxy at three temperatures.
From Horie et al., J. Polym. Sci., Polym. Chem. Ed. 8, 1357 (1970).
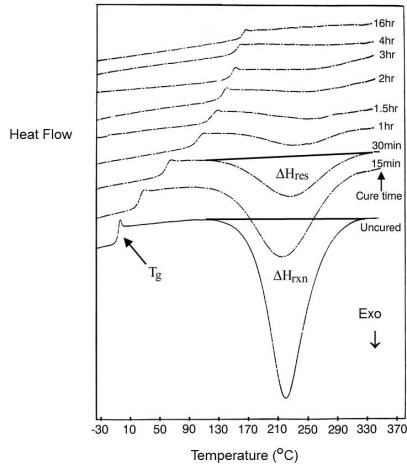
In Isothermal Method 2 individual samples are cured for varying times at each temperature, usually in an oven, and then scanned in the DSC to measure residual cure, as illustrated in the figure below. Conversion is calculated from the residual exotherm DHres as
Note that the rate of conversion da/dt is not measured in this method.
Epoxy-amine system cured for various times at 160°C.
From Wisanrakkit and Gillham, J. Appl. Poly. Sci 42, 2453 (1991).
The isothermal DSC methodsdescribedprovide the input for rate equations like Eqs. 3 and 4 in Part 1 of this series from which, as we will describe in future posts, rate constants k and reaction orders may be obtained. From Eq. 2 in Part 1 the activation energy E may be measured from a plot of ln k vs. T-1 at three or more temperatures.
To summarize we learned that autocatalytic reactions can be discerned from nth order reactions from the shape of an isothermal DSC curve. And we learned two isothermal DSC methods for measuring conversion (both Methods 1 and 2) and rate of conversion (Method 2) which, when input into the rate equation and the Arrhenius equation, can provide a complete description of the cure kinetics. In the next post we present time-temperature superposition (TTS) kinetics.

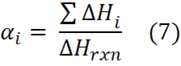
![clip_image002[6] clip_image002[6]](https://polymerinnovationblog.com/wp-content/uploads/2014/11/clip_image0026_thumb1.png)
![clip_image002[9] clip_image002[9]](https://polymerinnovationblog.com/wp-content/uploads/2014/11/clip_image0029_thumb1.png)
Leave a Reply