 In the last two posts, Dr. Prime discussed gelation and the relationship of functional group conversion at the gel point. Understanding the conversion at gelation has practical ramifications in many thermoset processes. As noted in a previous post, in the case of five minute epoxy, five minutes is the gel time. During thermoset processing, the ability to flow essentially stops at gelation. It is important to tailor your process to get the required flow prior to the gel point if you are processing a reactive thermoset such as an adhesive or prepreg.
In the last two posts, Dr. Prime discussed gelation and the relationship of functional group conversion at the gel point. Understanding the conversion at gelation has practical ramifications in many thermoset processes. As noted in a previous post, in the case of five minute epoxy, five minutes is the gel time. During thermoset processing, the ability to flow essentially stops at gelation. It is important to tailor your process to get the required flow prior to the gel point if you are processing a reactive thermoset such as an adhesive or prepreg.
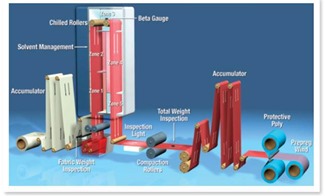
Let’s use prepregging as an example. In the image above (Courtesy of Hexcel Corporation (1)) the resin is dissolved in a solvent and impregnated into a woven fabric using metering rolls. The first side of the treater is for solvent removal. The zone temperatures are set to allow controlled solvent removal so that the moving web will not stick to the rollers in the turn around zone at the top of the treater. In the next zones the temperatures are set to provide the right amount of crosslinking reactions. As we have learned, thermosets typically start as small molecules and chain extension followed by crosslinking leads to a gelled network. Since prepreg needs to flow in subsequent processes (for example lamination or autoclave), the thermoset cannot be past the gel point.
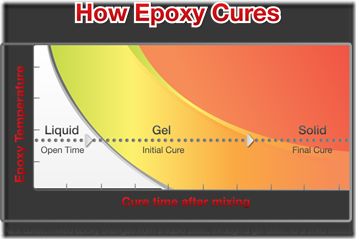
The choice of zone temperatures is critical since as can be seen in the following figure, the time to reach the gel curve shortens with increasing temperature:
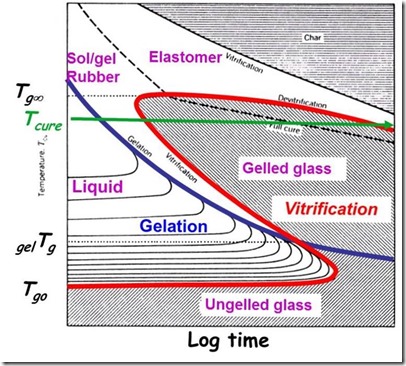
 In order to more fully understand the prepreg process, a very informative concept called the Time-Temperature-Transformation (TTT) diagram was pioneered by Prof. John Gillham at Princeton University (2). The following shows the TTT diagram for a typical epoxy:
In order to more fully understand the prepreg process, a very informative concept called the Time-Temperature-Transformation (TTT) diagram was pioneered by Prof. John Gillham at Princeton University (2). The following shows the TTT diagram for a typical epoxy:
Examination of the blue gelation curve one sees the same information as in the first figure, but expands to include more phase in the temperature, transformation (cure) and time region. Think of this as a dynamic phase diagram. The purple denotes the phases. One may see that at a temperature above Tgo the thermoset will be in the liquid region. In our prepregging example, we want to stay in this region during B-staging. B-staging is the term used for partially curing a thermoset (like an epoxy used in most composites) to increase the degree of cure (and Tg), but to control the crosslinking to stay below the gel point.
Another key aspect of the prepregging process is the ability to cool the partially cured (or B-staged) thermoset to below Tgo and transform the material to an ungelled glass. So let’s use the TTT diagram to understand the prepreg process.
- In the first two zones of the treater tower, the temperature is controlled to remove solvent with little chemical reactions occurring
- After the turn around zone, the temperatures are controlled to start the B-staging chemistry
- B-staging first results in chain extension as the epoxy groups undergo nucleophilic addition with hardeners or curing agents. The molecular weight begins to increase leading to higher viscosities
- As the B-staging continues, there may be limited crosslinking reactions as a result of the multifunctional epoxy or amine hardeners. The amount of crosslinking needs to be carefully controlled.
- At a give level of B-stage conversion, the thermoset impregnated web exits the treater tower and is quickly cooled. Since the thermoset has not gelled, upon cooling the material transforms into an ungelled glass (i.e not pas the gel point and no crystallization occurs)
- Typical conversion at the B-stage is in the range of 15-25%. Typical thermosets gel at conversions in the range of 35-50%, so we need to ensure the prepreg conversion is well below the gel conversion.
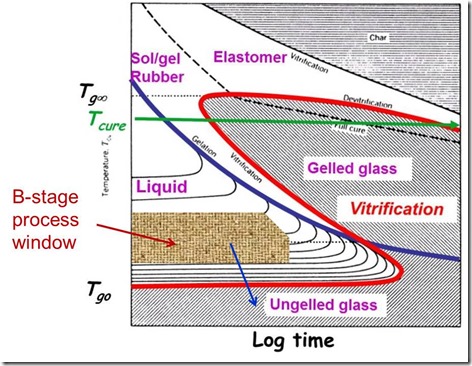
Let’s go back to the TTT diagram and examine the B-staging process window:
- The shaded area indicates the temperature and time region to allow the chemical reactions to cause chain extension and limited crosslinking. Remember we need to not advance too far toward the blue gelation curve.
- The blue arrow shows the effect of cooling on exiting the treater tower and the transformation of the prepreg into an ungelled glass.
- The reason we want an ungelled glass is that in subsequent composite processing, elevated temperatures are used to soften (heat above the prepreg Tg).
In the next several posts we will discuss the concept of vitrification during curing and processing of thermosets.
References:
- Hexcel Corporation, HexPly Prepreg Technology
- J. B. Enns and J.K. Gillham, J. Appl. Polym. Sci, 28, 2567 (1983)

Thanks for your invaluable posting. I have few questions.
1. What is the usual range of glass transition temperature of UNCURED epoxy (Tg0) ?
2. What are the boundary values of B-stage process window for Epoxy?
To be very specific I need to know those information for EPON 862-W resin system.
Looking for your reply.