Guest Post by Dr. R. Bruce Prime
 As a result of the chemical reactions discussed in earlier posts thermosets cross-link and become “set”. At a well-defined point in the cure reaction the thermoset transforms from a viscous liquid to a cross-linked gel or rubber, i.e. it gels.
As a result of the chemical reactions discussed in earlier posts thermosets cross-link and become “set”. At a well-defined point in the cure reaction the thermoset transforms from a viscous liquid to a cross-linked gel or rubber, i.e. it gels.
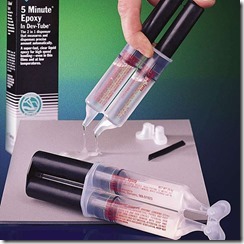
Gelation is the incipient formation of a cross-linked network, and it is the most distinguishing characteristic of a thermoset. A thermoset loses its ability to flow and is no longer processable above the gel point, and therefore gelation defines the upper limit of the work life. As an example, for a Five Minute Epoxy, which can be found in any hardware store, the five minutes refers to the gel point. (Image from Devcon www.devcon.com) After the two parts are mixed the user must form an adhesive joint within five minutes before the material becomes rubbery, and then keep the repaired part fixtured for several hours until cure is sufficiently complete.
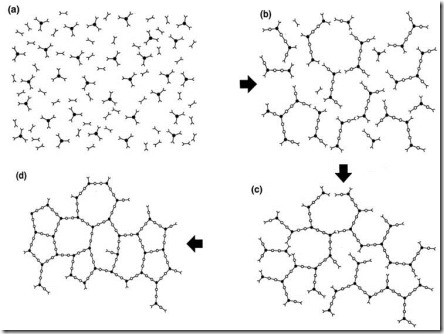
Figure 1. Schematic representation of cure and molecular gelation
Cure is illustrated schematically in Fig. 1 for a material with co-reactive monomers such as an epoxy-amine system. For cross-linking to occur at least one of the reactants must be trifunctional or higher. Reaction in the early stages of cure {(a) to (b)} produces larger and branched molecules and reduces the total number of molecules.
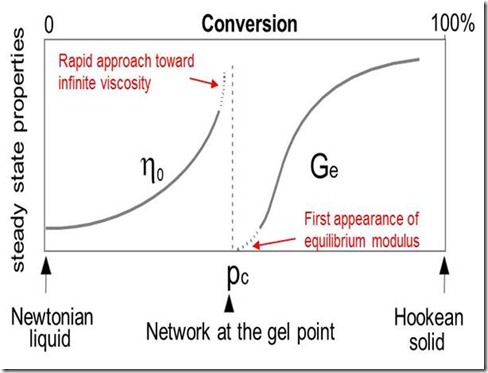
Macroscopically the thermoset can be characterized by an increase in its viscosity h (see Fig. 2 below). As the reaction proceeds the increase in molecular weight accelerates and all the chains become linked together at the gel point into a network of infinite molecular weight {approximately (c) in Fig. 1}. The gel point coincides with the first appearance of an equilibrium modulus as shown (which means it can support a load without flowing) in Fig. 2. Gelation does not affect the rate of cure and reaction continues beyond the gel point {(c) to (d)} to complete the network formation. Macroscopically, physical properties such as modulus and Tg build to levels characteristic of a fully developed network.
Figure 2. Macroscopic effects of cure including gelation
A distinction may be drawn between the phenomenon of molecular gelation and its consequence, macroscopic gelation. Molecular gelation occurs at a well-defined stage of the chemical reaction, provided the reaction mechanism is independent of temperature and free of non-cross-linking side reactions (1-3). Macroscopic consequences of gelation include a rapid approach toward infinite viscosity and development of elastic properties not present in the pregel resin. Molecular gelation may be detected as the point at which the reacting resin just becomes insoluble, or as the point where the mechanical loss tangent becomes frequency independent (4,5). Macroscopic means to approximate gelation include the time to reach a specific viscosity and the G’ = G” crossover in a dynamic rheology measurement.
In the next post we continue the discussion of gelation and show how conversion at the gel point agel, which is a constant for a given thermosetting system), can be calculated if the functionality and stoichiometry of the reactants are known.
References
1. P. J. Flory, J. Am. Chem. Soc. 63, 3083 (1941).
2. P. J. Flory, Principles of Polymer Chemistry, Cornell Univ. Press, Ithaca, NY (1953).
3. D. R. Miller and C. W. Macosko, Macromolecules 9, 206 (1976).
4. H. H. Winter, Polym. Eng. Sci. 27, 1698 (1987).
- M. Feve, Makromol. Chem., Macromol. Symp. 30, 95 (1989).

Nice article
Very beautifully and simply explain the matter
Thanks for sharing
Regards