Guest Post By Dr. R. Bruce Prime
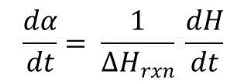 DSC can quantitatively measure the rate of cure, da/dt, from the exothermic heat flow, dH/dt, produced by the chemical cure reaction. As illustrated in the figure below, DHrxn is the total heat liberated from an uncured thermosetting system and can be measured in a DSC heating experiment. DH is expressed as heat per mole of reacting groups (kJ/mol or kcal/mole) or per mass of material (J/g or cal/g). The basic assumption underlying the application of DSC to cure is that the rate of the chemical cure reaction is proportional to the heat flow [the ordinate of a DSC measurement, measured in J/s (watts) or cal/s].
DSC can quantitatively measure the rate of cure, da/dt, from the exothermic heat flow, dH/dt, produced by the chemical cure reaction. As illustrated in the figure below, DHrxn is the total heat liberated from an uncured thermosetting system and can be measured in a DSC heating experiment. DH is expressed as heat per mole of reacting groups (kJ/mol or kcal/mole) or per mass of material (J/g or cal/g). The basic assumption underlying the application of DSC to cure is that the rate of the chemical cure reaction is proportional to the heat flow [the ordinate of a DSC measurement, measured in J/s (watts) or cal/s].
In cases such as the epoxy–amine reaction, it is further assumed that the heat of reaction for epoxy with primary amine is the same as that for the reaction of epoxy with secondary amine, and that the activation energies are the same. For systems with a symmetrical DSC exotherm these parameters are usually within a few percent of each other, providing that other thermal events are not occurring at the same time to interfere the cure exotherm. One example of an interfering event is the endothermic evaporation of residual solvent or, in the case of some phenolics and amino resins, water of condensation which occurs simultaneously with and as a result of the cure reaction.
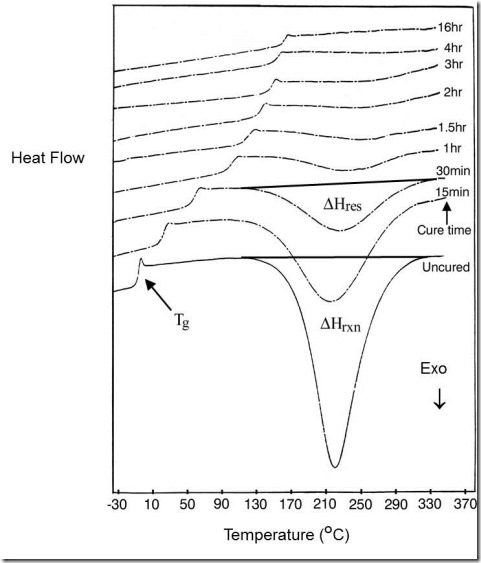
Figure 1. A series of DSC temperature scans at 10°C/min of an epoxy–amine cured isothermally at 160°C for different times, showing Tg increasing and the residual exotherm decreasing with increasing cure times. From Wisanrakkit and Gillham, J. Appl. Poly. Sci. 42, 2453 (1991).
In terms of a DSC measurement the conversion or degree of cure at time t, αt, is defined as:
where DHres is the residual heat of reaction at time t, also illustrated in Fig. 1.
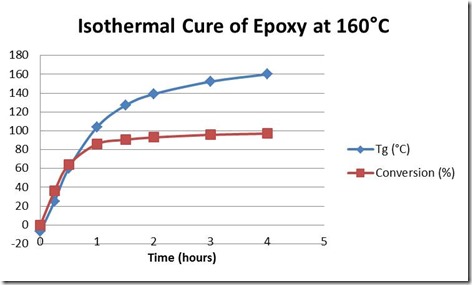
Figure 2 below illustrates the progress of cure for this epoxy-amine system in terms of the two parameters measured by DSC, conversion or degree of cure and the glass transition temperature.
Figure 2. Progress of cure as measured by Tg in °C and conversion from 0 to 100%. Note Tg for this system at 100% cure is 178°C. Same epoxy-amine as in Fig. 1.
The conversion or degree of cure increases rapidly to 90% after 2 hours but beyond that increases more slowly toward 100%. By comparison Tg undergoes a more rapid increase as it approaches 178C, the Tg at 100% conversion. This illustrates the greater ability of Tg to measure the state of cure near the completion of the reaction, especially when considering the diminishing cure exotherm.
In the next post we establish the relationship between Tg and conversion suggested by Fig. 1 and discuss in more detail the characterization of cure by DSC.

![clip_image002[7] clip_image002[7]](https://polymerinnovationblog.com/wp-content/uploads/2014/04/clip_image0027_thumb.png)
Hello, interesting post. I’ve tried to find the paper referenced at the images, but at that paper I didn’t found the images. Are you sure you got from Wisanrakkit and Gillham, J. Appl. Poly. Sci. 42, 2453 (1991)?
Kind regards,
I will check with Dr. Prime and see if he knows the answer to your question.