Guest Post by Dr. R. Bruce Prime
In the previous post we introduced the basic operating principles of TGA and illustrated some of the more common measurements. In this post we describe even more applications of TGA to thermosets.
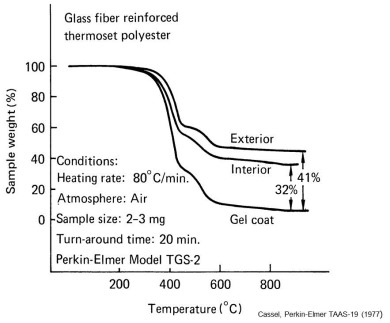
As demonstrated in the previous post TGA can quantitatively measure the composition of a thermally inert filler such glass fibers. In the above example we see that TGA at 80°C/min in air on small specimens can give a fast yet accurate measure of glass fiber content in a molded thermoset. Non-uniformity is observed between a sample from the interior versus one from the exterior. Unfilled gel coat is shown as a reference.
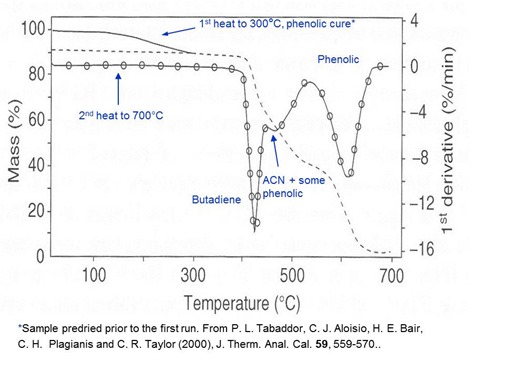
In the example above we see the application of TGA and DTG to an adhesive that is a blend of thermoplastic and thermoset. The thermoplastic is acrylonitrile (ACN)/butadiene (BD) and the thermoset is phenolic. The approximately 10% mass loss observed in the first heat is attributed to cure of the phenolic. In the second heat a three-step mass loss begins at ~450°C. Separate measurements showed that the first step was due to decomposition of butadiene, the second to decomposition of acrylonitrile plus some phenolic and the third to decomposition of phenolic. Thus this measurement gives a “fingerprint” that can be used to observe lot consistency in addition to a qualitative to semi-quantitative measurement of composition.
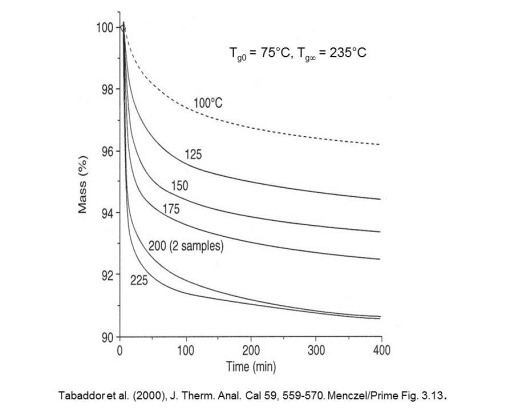
Here we observe the progress of cure for the same thermoplastic-thermoset adhesive from isothermal TGA at several temperatures between Tgo and Tg¥. Recall from Thermoset Characterization Part 7: Introduction to Vitrification posted on May 27, 2014 that when a thermoset is cured below its fully cured Tg or Tg¥ it will vitrify when the continuously increasing Tg becomes equal to the cure tempertature, i.e. at Tg = Tcure. The significant slowing of the reaction rate due to vitrification is evident at cure temperatures between 100 and 175°C. Only as the cure temperature approaches Tg¥ is >9% mass loss (i.e. full cure) achieved in a reasonable time. Data such as these provide the basis for kinetic analysis which will be the subject of future posts.
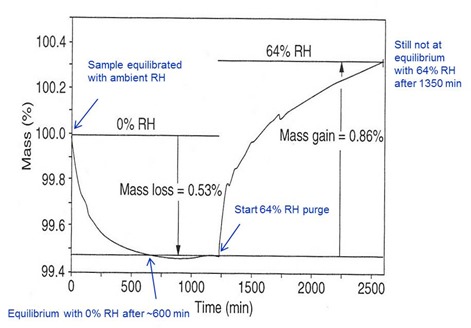
The final example illustrates the ability of TGA to measure moisture sorption and desorption on an epoxy thermoset. The measurement is made isothermally at lab ambient temperature. The measurement starts with a purge of 0% RH nitrogen on the sample that has been equilibrated at lab ambient (typically 23 to 25°C and 50% RH). The sample loses mass for about 600 minutes before stabilizing with a 0.53% mass loss. After 1250 minutes the purge gas is switched to nitrogen with 64% RH and the sample immediately begins to gain mass. A mass gain of 0.86% is observed after 1350 minutes but absorption is still occurring at an appreciable rate. Epoxies typically absorb between 1 and 3% moisture. Even this small amount can significantly reduce Tg and mechanical properties.
To recap, we saw that TGA can help optimize molding processes by measuring uniformity of filler content, characterize lot-to-lot variation of a thermoplastic/thermoset adhesive as well as provide time-temperature-extent of cure data for kinetic analysis and to aid process design, and characterize moisture sorption/desorption behavior of thermosets.

Leave a Reply