Guest Post by Dr. R. Bruce Prime.
 Differential scanning calorimetry or DSC is the most widely used analytical technique to characterize thermoset cure, due its ability to quickly and accurately measure the glass transition temperature Tg and conversion or degree of cure. Tg is the temperature where on heating the thermoset changes from a rigid glass to a liquid, or to a rubber if it has gelled. As we will see in later posts this information provides the experimental input to completely model the progress of cure, eg conversion = f(time and temperature). A good reference to DSC and the other thermal analysis techniques described in later posts is Thermal Analysis of Polymers: Fundamentals and Applications, (JD Menczel and RB Prime, eds), Wiley, 2009.
Differential scanning calorimetry or DSC is the most widely used analytical technique to characterize thermoset cure, due its ability to quickly and accurately measure the glass transition temperature Tg and conversion or degree of cure. Tg is the temperature where on heating the thermoset changes from a rigid glass to a liquid, or to a rubber if it has gelled. As we will see in later posts this information provides the experimental input to completely model the progress of cure, eg conversion = f(time and temperature). A good reference to DSC and the other thermal analysis techniques described in later posts is Thermal Analysis of Polymers: Fundamentals and Applications, (JD Menczel and RB Prime, eds), Wiley, 2009.
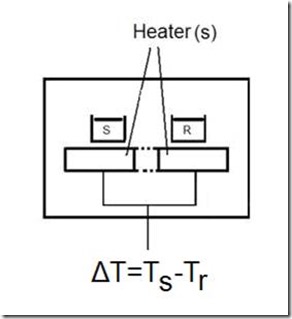
Typically 2 to 20 mg of material is placed in an aluminum capsule, shown above left, which is then placed on the sample (s) side of the DSC cell. Filled thermosets may require more than 20 mg in order to have sufficient resin for the measurement. An empty pan usually serves as the reference (r). There are two types of DSC. In a heat flux calorimeter, illustrated above, both sample and reference are heated uniformly so that the DT between sample and reference is a measure of the heat flow. In a power-compensation DSC, shown below, sample and reference are heated independently to maintain them at equal temperatures and the differential power is a measure of the heat flow. Heat flow into a material is endothermic, an example of which is the melting of a crystalline material that requires addition of the heat of fusion to melt the crystals. Heat flow out of a material is exothermic; thermoset cure is an example of an exothermic process. Note that in a DSC plot the endothermic direction can be either up or down.
DSC can measure the glass transition temperature of an amorphous material (Tg), and melting and crystallization of a crystalline material, as illustrated in Fig. 1.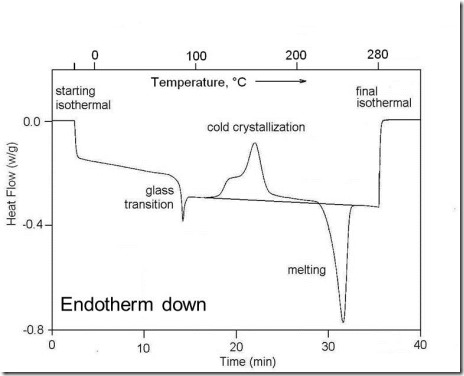
Figure 1. DSC at 10°C/min of amorphous poly(ethylene terephthalate) or PET. Observed in order of increasing temperature are the glass transition as a step change in the heat flow followed by the exothermic crystallization of the amorphous PET and finally the endothermic melting of the crystals formed during cold crystallization.
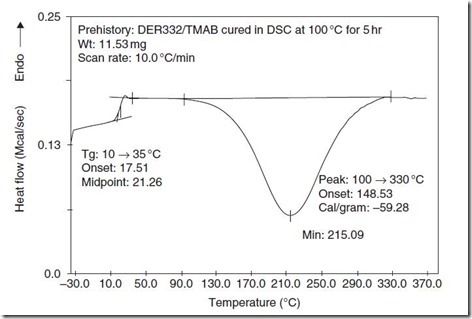
DSC can also measure the heat liberated in a chemical reaction, as illustrated in Fig. 2.
Figure 2. DSC at 10°C/min of epoxy-amine partially cured for 5 hours at 100°C. Observed are the glass transition at 21°C followed by the residual cure exotherm of 59 cal/g between 100 and 330°C. From Wisanrakkit and Gillham, J. Appl. Poly. Sci. 42, 2453 (1991).
As Fig. 2 suggests DSC has the ability to measure the advancement of cure for samples partially cured at a series of times and temperatures. It can also be used in the isothermal mode to measure conversion over the entire time spectrum at a given temperature.
In the next post we will explore the DSC of thermosets.

Leave a Reply