Guest Post by Dr. Robert Humphreys
 Transport any daring, young skateboarder back in time to the days before thermoset polyurethanes (PUs) and watch their enthusiasm for the sport dissipate as their knee and ankle joints respond to cold steel rolling on concrete and asphalt. Indeed, whether the application is skateboards, roller skates, suitcases, or forklifts, thermoset PU’s have revolutionized design and manufacture of wheels when light weight, vibration reduction, and abrasion resistance are critical to performance1.
Transport any daring, young skateboarder back in time to the days before thermoset polyurethanes (PUs) and watch their enthusiasm for the sport dissipate as their knee and ankle joints respond to cold steel rolling on concrete and asphalt. Indeed, whether the application is skateboards, roller skates, suitcases, or forklifts, thermoset PU’s have revolutionized design and manufacture of wheels when light weight, vibration reduction, and abrasion resistance are critical to performance1.
Image source: steel wheels and polyurethane wheels
Thermoset, or cross-linking, polyurethanes are employed in a broad range of applications where very tough, resilient, abrasion resistant or compression resistant materials are required, such as foams (flexible and rigid), structural adhesives, wheels, rollers, gears, and vehicle suspension bushings. They are one of the true engineering polymers. Thermoset PU technology can be based on aliphatic or aromatic monomers, depending on performance requirements. Aliphatic PU’s are particularly important when the application requires significant exposure to sunlight since aromatic PU’s are prone to UV-induced photo-oxidation2.
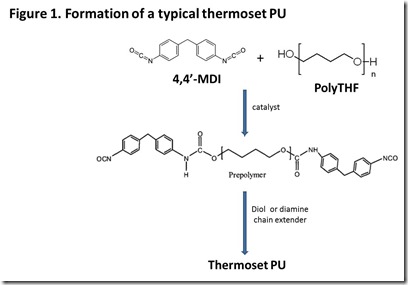
The chemistry of a typical aromatic thermoset PU is shown in Figure 1: a PU “prepolymer” is formed, followed by reaction with diol or diamine “chain extenders”. Properties of the cured thermoset are controlled by the choice of flexible “soft segment” polymers (polyTHF in Figure 1), type of isocyanate, ratio of isocyanate to hydroxyl functionality in the prepolymer, type of chain extender, and cure conditions. The large selection of isocyanate monomers, polyols, and chain extenders coupled with fillers and cure conditions affords the formulator with almost unlimited flexibility to tailor the properties of the thermoset PU to fit the application. It is beyond the scope of this post to discuss PU curing in detail, so the reader is referred to references for more information on PU chemistry and formulation3. This post will focus, instead, on the potential for making key components of commercial thermoset polyurethanes from renewable raw materials since there is great interest among users of these important engineering polymers in “greener” alternatives to the petroleum-based incumbents.
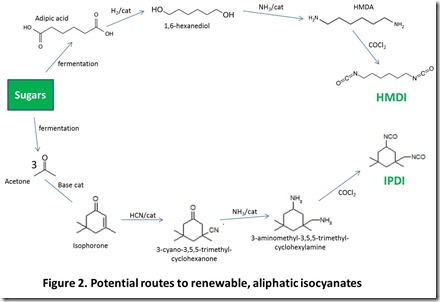
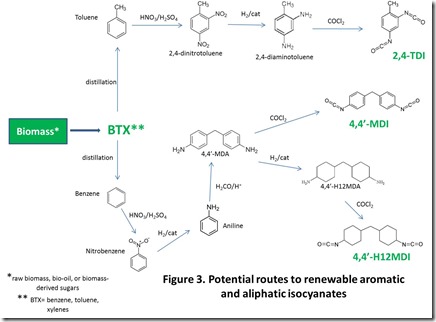
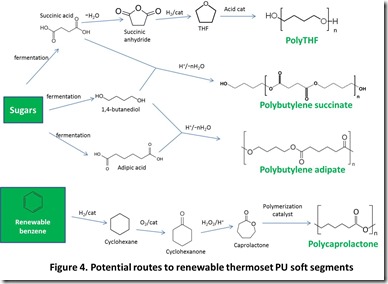
Potential renewable routes to popular aliphatic and aromatic isocyanates, polymeric diols, and chain extenders are shown in Figures 2, 3, 4, and 5 4.
Routes to renewable aliphatic isocyanates (Figure 2) probably have received the most attention because they can be prepared in a straightforward way from important renewable chemicals. For example, hexamethylene diisocyanate, or HMDI, can be prepared from hexamethylenediamine, or HMDA. HMDA is accessible from fermentation-derived adipic acid 5 by catalytic reduction to 1,6-hexanediol followed by amination to form HMDA and reaction of HMDA with phosgene6. Isophorone, a key precursor for isophoronediisocyanate (IPDI), can be prepared from fermentation-derived acetone by an old and well known condensation reaction; isophorone is then converted in a straightforward way to IPDI 7.
Renewable toluene-2,4-diisocyanate (2,4-TDI) and 4,4’-methylenediphenyl diisocyanate (4,4’-MDI) require renewable sources of toluene and benzene. BTX, or benzene/toluene/xylenes, can be prepared via pyrolysis of renewable materials such as sugars or biomass 8. Separation of BTX by distillation provides bio-benzene and bio-toluene. Bio-benzene can be converted into 4,4’-methylenediphenylamine (4,4’-MDA) by nitration to form nitrobenzene, catalytic reduction to aniline, and reaction of aniline with formaldehyde to give MDA. Bio-toluene can be converted to 2,4-diaminotoluene via nitration and catalytic hydrogenation of the nitro groups (see Figure 3). Conversion of 4,4’-MDA to 4,4’-MDI and 2,4-toluenediamine to 2,4-TDI is accomplished with phosgene. Renewable aliphatic 4,4’-H12MDI can also be prepared via reaction with phosgene with bio-sourced 4,4’-MDA 9 (also shown in Figure 3).
Most of the attractive properties of thermoset polyurethanes, such as high impact and abrasion resistance and low temperature flexibility, can be attributed to phase separation into a “hard,” or polyurethane-rich phase and a “soft,” or flexible segment-rich phase 10. The flexible segment-rich phase can be generated by incorporation of a variety of polyester and polyether diols. Examples of polyester diols include: poly(butylene succinate) and poly(butylene adipate), which can be prepared from renewable succinicand adipic acids and butane-1,4-diol; and polycaprolactone 11, which can be prepared from renewable benzene (see BTX discussion above). A commonly employed polyether diol is polytetrahydrofuran, or polyTHF12. Renewable polyTHF can be prepared from bio-sourced succinic acid. These chemical transformations are illustrated in Figure 4. It is worth noting that both 1,4-butanediol and THF can be prepared from succinic acid, which is an indication of why succinic acid is a popular renewable chemical target.
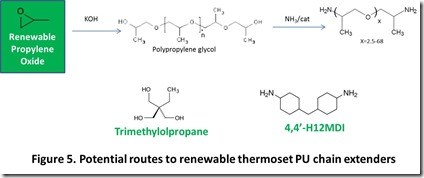
Finally, Figure 5 provides the structures of three important thermoset PU chain extenders: trimethylolpropane, 4,4’-MDA, and Jeffamine® polyetheramines 13. Renewable trimethylolpropane was discussed in a previous post14. A potential route to Jeffamine® polypropylenediamines is shown in Figure 5; renewable propylene oxide was also discussed in a previous post15.
We conclude this post by pointing out that routes to renewable versions of key thermoset PU monomers and chain extenders described above employ well known process chemistry starting from renewable versions of bulk chemicals such as benzene, toluene, acetone, formaldehyde, succinic acid, adipic acid, 1,4-butanediol, propylene oxide and phosgene. In other words, existing chemical processes can be employed once renewable versions of these bulk chemicals are available. It may seem somewhat outrageous to refer to “renewable phosgene”, yet phosgene is produced from the reaction of carbon monoxide with chlorine gas and renewable CO is available from syn gas (CO +H2) that can be generated from biomass16. While it may be far into the future, even manufacture of “green” chlorine gas (pun intended) is potentially achievable if the electricity used in the sodium chloride electrolysis process is generated from renewable sources17!
The next post in this series about potential renewable routes to thermoset polymers will focus on phenolic resins, which also will provide a good introduction to the following post on epoxy resins.
References:
1. a) See Dipak Parekh, “What are castable urethane elastomers? ”, reference1 .
b) “PolyTHF: Supporting your growth”, BASF Intermediates.
2. a) “Polyurethane types”, Marquinsa, see https://www.merquinsa.com/whats/FPPUtypes1.pdf ; b) Dan Rosu and Liliana Rosu, “Thermal and photochemical stability of an aromatic polyurethane”, Materiale Plastice, 2010, 47, 399-404, available at reference 2b .
3. a) Ronald W. Fuest, “Polyurethane Elastomers”, Chapter 9 in Rubber Technology: Compounding and Testing for Performance, 1st Ed, John S. Dick, editor, Hansen Gardner Publications, 2001, available at reference 3a; b) See reference 1b; c) “Flexible polyurethane foam chemistry”, available at reference 3b ; d) Dennis G. Lay and Paul Cranley, “Polyurethane Adhesives”, Chapter 34 in Handbook of Adhesive Technology, 2nd Edition, available at reference 3d ; e) Paul Fitzgerald and Andrew Davies, “Polyurethane cast elastomers: a natural extension for rubber moulders”, available at reference 3e ; f) LyondellBasell PolyMEG Polyols, available at https://www.lyondellbasell.com/techlit/techlit/2268.PDF .
4. Structure images from Wikipedia, Wikimedia, Sigma-Aldrich.com for [4,4’-methylene-bis(cycohexylamine)], polybutylene adipate; IPDI images in Figure 2 from reference 7a; www.sciencedirect.com for polybutylene succinate; Jeffamine image from reference 13a; Figure 1 prepolymer image from iopscience.iop.com.
5. A number of companies have announced intent to commercialize bio-adipic acid: reference 5 .
6. a) US patent 6,426,438, “Method for producing 1,6-hexanediol and 6-hydroxycaproic acid or their esters”; b) US patent 3,268,588, “Process for producing hexamethylenediamine from 1,6-hexamediol”; c) US patent 5,136,086, “Preparation process of aliphatic isocyanate”, US patent 4,922,005, “Preparation process of hexamethylene diisocyanate”.
7. a) See reference 7; b) see US patent 4,535,187, “Preparation of Isophorone and mesityl oxide from acetone”.
8. a) For a review of BTX production from biomass, see David Dodds and Bob Humphreys, “Production of aromatic chemicals from biobased feedstock”, Chapter 8 in Catalytic Process Development for Renewable Materials, eds. Pieter Imhoff and Jan Cornelis van der Waal, Wiley-VCH, Germany, 2013, and references cited; b) US patent 7,977,517, “Synthesis of liquid fuels and chemicals from oxygenated hydrocarbons”; c) Tushar P. Vispute, Huiyan Zhang, Aimaro Sanna, Rui Xiao, and George W. Huber, “Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils”, 2010, Science, 330, 1222-1227, available at reference 8c.
9. US patent 3,856,862, “Process for hydrogenation of di(4-aminophenyl)methane with a rhodium catalyst”.
10. See reference 3e for a good overview of phase separation and PU properties.
11. For a comprehensive list of companies working on renewable chemicals, see Table 1.2 in Leo E. Manzer, Jan Cornelis van der Waal, and Pieter Imhof, “The Industrial Playing Field for the Conversion of Biomass to Renewable Fuels and Chemicals”, Chapter 1 in Catalytic Process Development for Renewable Materials, eds. Pieter Imhoff and Jan Cornelis van der Waal, Wiley-VCH, Germany, 2013, and references cited.
12. See ref 1b for information about polyTHF.
13. a) “The Jeffaine® Polyetheramines”, b) See, for example, US patent 4,766,245, “Process for the preparation of polyalkoxyalkylene polyamines”.
14. See the July 2, 2013 post, https://polymerinnovationblog.com/ .
15. See July 8, 2013 post, https://polymerinnovationblog.com/ .
16. See Section 3.1 in Yu-Chuan Lin and George W. Huber, “The critical role of heterogeneous catalysis in lignocellulosic biomass conversion”, 2009, Energy and Environmental Science, 2, 68-80.
17. Wolf H. Hilbertz, “Solar-generated construction material from sea water to mitigate global warming”, 1991, Building Research & Information, 19, 242-255, available at reference 17

Leave a Reply