Guest Post by Dr. Robert Humphreys
 If your idea of a relaxing day is cruising on the lake in your boat, chances are you appreciate the properties of “fiberglass” construction. Fiberglass is light weight, strong, and easily formed into a wide range of shapes. Besides pleasure boats, applications include body panels for automobiles and trucks, bathtubs and shower stalls, corrugated roofing, entry door skins, wall panels for buildings, and many others where the high strength-to-weight ratio, durability, and low cost are important.
If your idea of a relaxing day is cruising on the lake in your boat, chances are you appreciate the properties of “fiberglass” construction. Fiberglass is light weight, strong, and easily formed into a wide range of shapes. Besides pleasure boats, applications include body panels for automobiles and trucks, bathtubs and shower stalls, corrugated roofing, entry door skins, wall panels for buildings, and many others where the high strength-to-weight ratio, durability, and low cost are important.
Fiberglass is a composite of glass fibers embedded in a matrix of thermoset unsaturated polyester (UP) resin, although other resins are sometimes employed 1. The glass fibers can be in different forms, such as woven fabric or mats onto which resin is spread or chopped fibers uniformly dispersed in UP resin. A variety of fillers can also be used to augment resin properties and reduce cost 2. Sheet molding compound (SMC) and bulk molding compound (BMC) are typical forms of formulated UP resins used to manufacture molded articles 3.
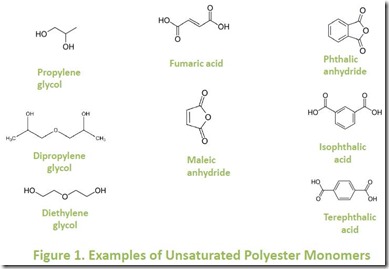
Unsaturated polyester (UP) resins are prepared by condensation polymerization of glycols with unsaturated dicarboxylic acids. The resulting unsaturated polymers can be cross-linked, or cured, using free radical reactions generated thermally using initiators such as peroxides 4, photochemically, or by e-beam. Typical glycol and dicarboxylic acid monomers used in UP resins are illustrated in Figure 15.
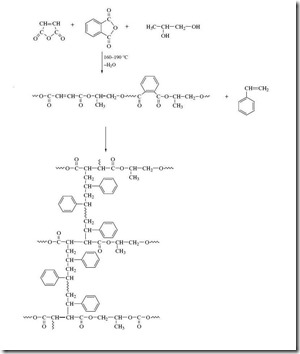
Unsaturated polyester resins are usually formulated with a significant amount (up to 60%) of a vinyl monomer such as styrene. Formation and curing chemistry of a prototypical UP resin is shown in Figure 2 5. Starting monomers are maleic anhydride, phthalic anhydride, and propylene glycol to build the unsaturated polyester. Styrene (with free radical initiator) is added as a crosslinker in this example:
Figure 2: Formation and Curing of UP Resin
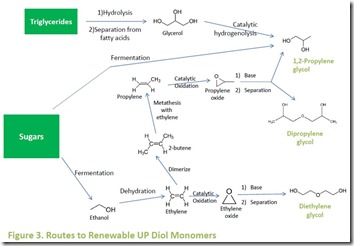
All of the monomer components illustrated in Figure 1 can be made from renewable raw materials. Potential and existing bio-routes to UP diols are shown in Figure 3 5. The diol 1,2-propylene glycol can be produced directly by fermentation of carbohydrates such as sugars 6, from vegetable oil derived glycerol by catalytic hydrogenolysis 7, or from fermentation-derived ethanol. Ethanol is dehydrated to ethylene8, followed by catalytic dimerization to 2-butene9, and metathesis of 2-butene with ethylene to yield propylene10. Propylene can then be oxidized to propylene oxide, which can be converted to 1,2-propylene glycol, dipropylene glycol, and polypropylene glycols11. Fermentation-derived ethanol is also the bio-source for renewable diethylene glycol , as discussed in the July 2, 2013 post on this blog.
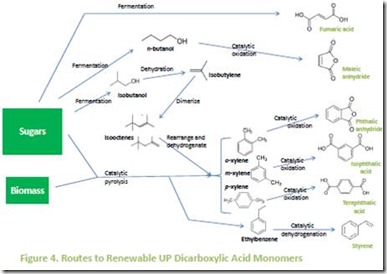
Bio-routes to UP dicarboxylic acids are shown in Figure 4 12. Fumaric acid is a direct product of fermentation of sugars such as glucose13. Maleic anhydride can be made by catalytic oxidation of fermentation-derived n-butanol 14 or catalytic oxidation of furfural, a product of acid-catalyzed rearrangement of 5-carbon sugars such as xylose 15. There appears to be little effort at this point to develop a commercial process for this maleic anhydride since succinic acid can be converted to succinic anhydride and substituted for many of the applications that traditionally rely on maleic anhydride, such as conversion to tetrahydrofuran, 1,4-butanediol and γ-butyrolactone. However, succinic anhydride cannot replace maleic anhydride in UP applications.
The aromatic dicarboxylic acid UP monomers (phthalic, isophthalic and terephthalic) can be made from renewable sources via fermentation or chemical conversion processes. Pyrolysis of biomass or sugarsderived from biomass can generate renewable ethylbenzene along with ortho-, meta-, and para-xylene16. Figure 4 also illustrates chemistry for conversion of isobutanol to a mixture of xylenes and other aromatics 17. Ethylbenzene can be catalytically dehydrogenated to styrene18 while ortho-, meta-, and para-xylene can be catalytically oxidized to phthalic acid or anhydride, isophthalic acid and terephthalic acid, respectively19. The reader should note that industrial processes already exist for converting petroleum-derived ethylbenzene to styrene and xylenes to the corresponding dicarboxylic acids, so renewable ethylbenzene and xylenes should be “drop-in” replacements for the manufacture of renewable styrene and aromatic dicarboxylic acid UP monomers in existing manufacturing facilities.
The previous post pointed out that many chemicals that are building block molecules for the petrochemical industry are targets of the renewable chemicals industry and this is certainly the case for the renewable chemicals used to make important UP monomers, such as the xylenes, ethyl benzene, butanol, and propylene oxide. So, if you haven’t purchased that special boat you’ve always wanted, perhaps by the time you make up your mind, one of your options may be a choice between “oil-based” or “renewable” fiberglass.
In the next post in this series, we will focus on routes to renewable thermoset polyurethanes.
References:
- Hui Li, “Synthesis, characterization, and properties of vinyl ester matrix resins”, Ph.D. dissertation, 1998, University of Vermont. Chapter 1, available at Reference 1
- Johannes Karl Fink , “Unsaturated Polyester Resins”, Chapter 1 in Reactive Polymers Fundamentals and Applications, William Andrew Publishing, Norwich, NY, 2005. See https://pharosproject.net/uploads/files/cml/1336053249.pdf .
- “Microthene®F: Powders for SMC/BMC Applications”, available at Reference 3
- For a list of peroxide and other initiators, see reference 2, page 36.
- Fig.1 structures taken from images at Wikipedia, pesticideinfo.org and sciencedirect.com. Fig. 2 UP resin curing illustration taken from “unsaturated polyester images” at intechopen.com .
- a) See World patent application WO2010/051849, “Use of sucrose as substrate for fermentive production of 1,2-propanediol”; b) D.C.Cameron, N.E.Altaras, M.L.Hoffman, and A.J.Shaw, “Metabolic engineering of propanediol pathways”, Biotechnology Progress, 1998, 14, 16-12; c) Nedim E. Altaras and Douglas C. Cameron, “Enhanced production of (R)-1,2-propanediol by metabolically engineered Escherichia coli”, Biotechnology Progress, 2000, 16, 940-946; d) G.N.Bennett and K.-Y. San, “Microbial formation, biotechnological production and applications of 1,2-propanediol”, Applied Microbial Biotechnology, 2001,55, 1-9.
- a) reference 7a ; b) reference 7b ; d) Mohanprasad A. Dasari, Pim-Pahn Kiatsimkul, William R. Sutterlin, and Galen J. Suppes, “Low pressure Hydrogenolysis of glycerol to propylene glycol”, Applied Catalysis A: General, 2005, 281, 225-231, available at https://www.epa.ohio.gov/portals/41/p2/wasteglycerol.pdf ; e) M. Olga Guerrero-Perez, Juana M. Rosas, Jorge Bedia, Jose Rodriguez-Mirasol, and Tomas Cordero, “Recent inventions in glycerol transformations and processing”, Recent Patents in Chemical Engineering, 2009, 2, pages 11-21.
- a) https://www.scidesign.com/index.php?id=19 ; b) reference 8b ; c) Denise Fan, Der-Jong Dai, and Ho-Shing Wu, “Ethylene formation by catalytic dehydration of ethanol with industrial considerations”, Materials, 2013, 6, 101-115, see https://www.mdpi.com/1996-1944/6/1/101 .
- See US patent 4,242,31, “Olefin Dimerization”.
- a) Shengjun Huang, Shenlin Liu, Wenjie Xin, Jie Bai, Sujuan Xie, Qingxai Wang, and Longya Xu, “Metathesis of ethane and 2-butene to propene on W/Al2O3-HY catalysts with different HY contents”, J. Molecular Catalysis A: Chemical, 2005, 226, 61-68; b) reference 10b ; c) Masakazu Iwaamoto, “One step formation of propene from ethene or ethanol through metathesis on nickel ion-loaded silica”, Molecules, 2011, 16, 7844-7863, see https://www.ncbi.nlm.nih.gov/pubmed/22143546 d) see reference 10d.
- “Alton E. Martin and Frank H. Murphy, “Glycols Propylene Glycols”, see Reference 11 .
- Figure 4 credits
- a) US patent 2,861,922, “Fumaric acid fermentation process”; b) US patent 4,877, 731, “Fermentation process for carboxylic acids”.
- a) V.V.Guliants, J.B.Benziger, and S.Sundaresan, “The oxidation of C4 molecules on vanadyl pyrophosphate catalysts”, in Studies in Surface Science and Catalysis, J.W.Hightower, W.N.Dellgass, E. Iglesia, and A.T.Bell, eds., Vol. 101, 991-1000. Elsevies Science B.V., 1996; b) US patent application 2012/0015411, “Manufacture of maleic anhydride from renewable materials, maleic anhydride obtained and uses thereof”.
- Noelia Alonso-Fagundez, Manuel Lopez Granados, Rafael Mariscal, and Manuel Ojeda, “Selective oxidation of furfural to maleic anhydride with VOx/Al2O3 catalysts”, 2012, ChemSusChem, 1984-1990.
- David Dodds and Bob Humphreys, “Production of aromatic chemicals from biobased feedstock”, Chapter 8 in Catalytic Process Development for Renewable Materials, eds. Pieter Imhoff and Jan Cornelis van der Waal, Wiley-VCH, Germany, 2013.
- a) US patent application 2012/0171741, “Renewable xylenes produced from biological C4 and C5 molecules”; b) David A. Glassner, “Hydrocarbon Fuels from Plant Biomass”, available at Advanced Biofuels .
- a) . Coulter, D.W.Goodman and R.G.Moore, “Kinetics of the dehydrogenation of ethylbenzene to styrene over unpromoted and K-promoted model iron oxide catalysts”, 1995, Catalysis Letters, 31, 1-8; b) US patent 6,096,937, “Process for dehydrogenation of ethylbenzene to styrene”.
- US patent 4,172,209, “Process for producing dicarboxylic acids by the oxidation of xylene”.


Very important to appoint the renewable source from nature. We must think if or when oil source could be “disappeared” .