Guest Post by Dr. Robert Humphreys
Thermoset polymers are part of every moment of our lives. Modern electronics, including computer chips, cell phones, tablets and laptops, servers, GPS devices, calculators, medical devices, and touch-pad controls, would be impossible without thermosets. Modern home and building construction depends on materials based on thermoset polymer technology. Thermosets are critical to the manufacture of automobiles, airliners such as the Boeing 787 and the Airbus 350, most sports equipment, furniture, appliances, military equipment and weapons…the list is virtually endless. Even your dentist relies on thermoset polymers to repair or replace your teeth.
A vast array of thermoset polymer technologies is available commercially. On the other hand, five thermoset technologies:
- Acrylics
- Unsaturated polyesters
- Epoxies
- Polyurethanes
- Phenolics
are so common that they are encountered directly or indirectly by virtually everyone each day. All five of these technologies are derived from petroleum and, therefore, are not based on renewable resources. Nevertheless, it is possible to produce all of these polymers from renewable resources. This and the next four posts will outline some of the potential routes to the “Big Five” thermoset polymers from renewable raw materials, including information on commercialization where it is available. We will begin this journey with acrylic thermosets.
Renewable Acrylic Thermosets
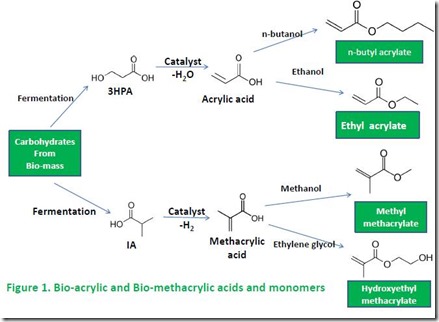
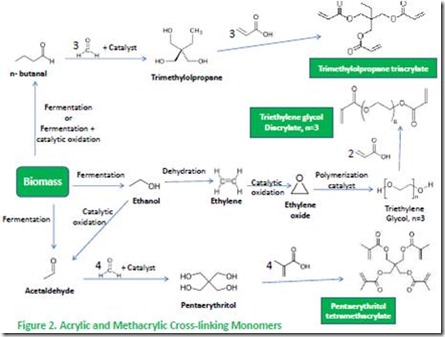
Acrylic thermosets are employed in many commercial applications, including dental cements and composites, anaerobic and structural adhesives, superabsorbent polymers, and kitchen countertops1. Acrylic thermosets include technologies based on either acrylic acid or methacrylic acid. Ethyl acrylate, butyl acrylate, hydroxyethyl methacrylate, and methyl methacrylate are examples of common mono-functional monomers. Triethylene glycol diacrylate, trimethylolpropane triacrylate and pentaerythritol tetramethacrylate are examples of di-, tri-, and tetrafunctional monomer cross-linking agents. Structures of these monomers and some of the raw materials used to make them are illustrated in Figures 1 and 22.
Replacing petroleum-derived acrylic thermosets with equivalent, renewable versions requires bio-sourced acrylic acid, methacrylic acid, and diols, polyols and other multifunctional molecules used to make cross-linking monomers. Renewable acrylic acid has received the most attention. Renewable acrylic acid can be prepared by catalytic dehydration of 3-hydroxypropionic acid (3HPA) or lactic acid (LA); both 3HPA and LA are prepared by fermentation of carbohydrates3a. LA dehydration to acrylic acid is prone to formation of other products3b, such as lactide (the precursor for polylactic acid, or PLA, production), so dehydration of 3HPA is a superior route3c. The list of potential bio-acrylic acid producers is extensive3d.
Reports of renewable methacrylic acid production are less common4a. Isobutyric acid (IA) can be dehydrogenated to methacrylic acid4b; IA can be produced by fermentation using an appropriately engineered organism5.
Most monofunctional monomers employed in acrylic thermosets are ester derivatives of acrylic acid or methacrylic acid with alcohols such as methanol, ethanol, and butanol (see Figure 1). Simple alcohols such as ethanol and n-butanol can be produced from carbohydrates such as sugars by fermentation by organisms such as engineered yeast6. Methanol can be produced from biomass-derived synthesis gas (carbon monoxide, or CO, and hydrogen, or H2)7. Fermentation-derived ethanol is also the source of ethylene glycol: ethanol is dehydrated to ethylene8, followed by catalytic oxidation to ethylene oxide9. Ethylene oxide can then be converted to ethylene glycol or to polyethylene glycol (PEG) oligomers and polymers8,9. Conversions of ethylene to ethylene oxide and ethylene oxide to ethylene glycol and PEG are practiced on a huge scale by the petrochemical industry, obviating the need to develop processes for converting bio-ethylene (i.e. bio-ethylene is a drop-in replacement for petro-ethylene).
Cross-linking monomers such as those shown in Figure 2 are necessary to produce thermoset acrylic resins. Polyethylene glycol oligomers, trimethylolpropane, and pentaerythritol provide low cost substrates for preparation of the respective di-, tri-, and tetrafunctional acrylic and methacrylic cross-linking monomers. We have already described the process for conversion of ethanol to ethylene oxide. Trimethylolpropane is prepared by condensation of the aldehyde n-butanal10, which can be prepared directly from fermentation of carbohydrates, with formaldehyde11. Finally, pentaerythritol can be prepared by condensation of bio-sourced acetaldehyde12 with formaldehyde13.
It may seem from this discussion that replacing petrochemical-based acrylic monomer technology with bio-sourced, “drop-in” equivalent materials will be a long and winding road. However, optimists will note that the major building blocks for the monomers illustrated in Figures 1 and 2 are based on bulk chemicals such as methanol, ethanol, ethylene, ethylene oxide, ethylene glycol, formaldehyde, acetaldehyde, n-butanol, n-butanal, isobutanol, and acrylic acid. All of these molecules are accessible from renewable raw materials and most are targets of the renewable chemicals industry. Thus, as the renewable chemicals industry continues to develop and these chemicals become available in quantity at low cost, renewable thermoset acrylic technology should not be far behind.
In the next post in this series, we will focus on renewable thermoset unsaturated polyester technology.
References:
- For dental cements, see: Dental Cements ; b) for an overview of superabsorbent polymers, see: Superabsorbent polymers ; for information on acrylic composite kitchen countertop technology, see US patent 3,405,088.
- Structures used in Figures 1 and 2 taken from Wikipedia (acrylic acid, methacrylic acid, isobutyric acid, 3-hydroxypropanoic acid, ethyl acrylate, methyl methacrylate, trimethylolpropane, pentaerythritol), glycosan.com (polyethylene glycol diacrylate), and polyscience.com (polyethylene glycol dimethacrylate, trimethylolpropane trimethacrylate), synasis.com (trimethylolpropane triacrylate), marketpublishers.com (pentaerythritol tetramethacrylate), chemicalbook.com (pentaerythritol tetraacrylate).
- a) US patent application 2011/0125118A1; b) Xu Xiaaobo, Lin Jiaanping, Cen Peilin, “Advances in research and development of acrylic acid production from biomass”, Chinese J. Ch. E. , 2006, 14, 419-427, see Reference 3a ; c) Andrew Cie, Roy Schlarp, Stephen Lantz, Metaxia Tzakas, “Renewable Acrylic Acid”, Department of Chemical and Biochemical Engineering senior design report, University of Pennsylvania, see https://repository.upenn.edu/cgi/viewcontent.cgi?article=1038&context=cbe_sdr; d) For a list of companies that have announced plans for bio-acrylic acid, see “From diapers to paints- is bio-acrylic acid on the way”, a prospectus by Nexant
- a) See Reference 4a and Ascenix Biotechnologies report in Reference 4a2 ; b) Emilie Blouet-Crusson, Monique Rigole, Michel Fournier, Antoine Aboukais, Franck Daubrege, Gerhard Hecquet, and Michel Guelton, “Oxidative dehydrogenation of Isobutyric acid with cupric salts of molybdovanadophosphoric acid (CuxH4-2xPVMo11O40). Investigation of catalyst activation and deactivation”, Applied Catalysis A: General, 1999, 178, 69-83.
- See world patent WO2012/109534A2.
- See Peter H. Pfromm, Vincent Amanor-Boadu, Richard Nelson, PraveenVadlani, and Ronald Mandl, “Bio-butanol vs bio-ethanol: a technical and economic assessment for corn and switchgrass fermented by yeast or Clostridium”, Biomass and Bioenergy, 34, 515-524, available at Reference 6 .
- Kim Aasberg-Petersen, Charlotte Stub Nielsen, Ib Dybkjaer, and Jens Perregaard,” Large scale methanol production from natural gas”, Haldor Topsoe, see Reference 7 .
- 8. a) Reference 8a ; b) Reference 8b ; c) Denise Fan, Der-Jong Dai, and Ho-Shing Wu, “Ethylene formation by catalytic dehydration of ethanol with industrial considerations”, Materials, 2013, 6, 101-115, see https://www.mdpi.com/1996-1944/6/1/101 .
- Siegfried Rebsdat and Dieter Mayer, “Ethylene Oxide” in Ullman’s Encyclopedia of Industrial Chemistry, Vol. 13, 547-572, Wiley-VCH, Weinheim, Germany, 2012.
- See US patents 4,514,578 and 7,211,701B2.
- The most likely source of renewable formaldehyde is via oxidation of bio-sources methanol. See, for example, Mohammed Ahmad Sanhoob, Abdullah Al-Sulami, Fawaz Al-Shehri, and Sabil Al-Rasheedi, “Production of formaldehyde from methanol”, and references cited
- a) See world patent WO2009/120796; b) N. Iguchi, T.Takei, and M.Haruta, “Synthesis of acetaldehyde and acetic acid by the gas phase oxidation of ethanol over gold and vanadium catalysts”
- H.B.J. Schurink, “Pentaerythritol”, Organic Synthesis, 1941, Coll. Vol. 1, 425-427.



Leave a Reply