Guest Post by Dr. Robert Humphreys
In previous posts, we have alluded to the immense complexity of the initiative to replace petroleum with biomass as the principal source of transportation fuels, chemicals, and polymer-based materials. Biomass pretreatment, the 5th step in the biomass-to-fuels-and-chemicals chain illustrated in Figure 1, provides perhaps the best example of the multifaceted nature of this challenge. There are many options for pretreatment, depending on the technology that will be used to convert the biomass into marketable fuels, chemicals, and polymers. We don’t have room here to expound upon all pretreatment technologies, so we will outline them and provide readily accessible reference links for readers who want more information. 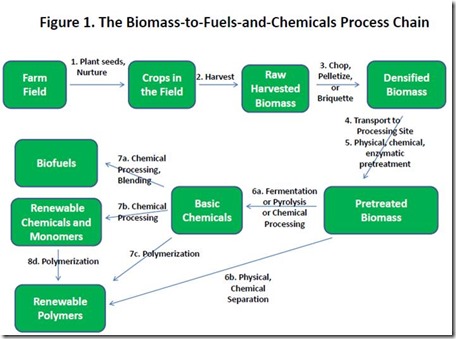
Drying
Freshly harvested biomass invariably has a moisture content that is high enough to adversely impact storage stability and further processing . Drying is usually necessary, with the final moisture content specification depending on requirements of further processing. Drying costs rise as final moisture content specification drops. Capital and operating costs for various drying technologies have been estimated (Biomass drying technology).
Mechanical Pretreatments
Mechanical pretreatment is usually the next step in conversion of any biomass to fuels and chemicals. Mechanical pretreatment can involve chopping, compacting to improve the economics of transport, or grinding to a fine particle size. Particle size can be critical to further processing, with particle sizes as small as 0.2-6.0 mm for fast pyrolysis processes (Particle size 1, see page 462) and 0.5-3.0 mm for corn stover ethanol production (Particle size 2, page 505). For example, in fast pyrolysis, which relies on a very short residence time in the hot zone, small particle size is crucial to rapid, uniform heating of the biomass to the pyrolysis temperature. Particle size reduction can add significantly to feedstock cost (Particle size 2, page 511).
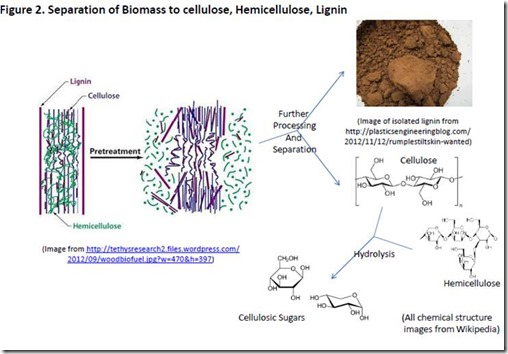
Separation of Cellulose, Hemicellulose, and Lignin
Biomass composition depends on source, with most sources falling in the range of 30-55% cellulose and 25-50% hemicellulose (biomass composition), with the remainder predominantly lignin. While some processes for conversion of biomass to fuels and chemicals utilize unseparated biomass (e.g. fast pyrolysis), other processes require separation of lignin from the carbohydrates and hydrolysis of the cellulose/hemicellulose fraction to sugars (e.g. fermentation, some pyrolysis methods).
The pulp and paper industry developed technology for separating cellulose from biomass long ago and has since improved the efficiency of the process to the point where modern manufacturing facilities are virtually zero effluent and generate more energy than they consume ( pulp and paper). The dissolving pulp process can produce almost pure cellulose.
Combinations of mechanical, thermal and chemical treatments can achieve almost complete lignin removal, along with varying degrees of hemicellulose and cellulose separation. A partial list of such treatments includes:
- Steam or ammonia explosion. Lignocellulosic biomass (LB) is exposed to steam or ammonia under pressure, followed by sudden release of the pressure that results in disruption of the biomass fiber structure.
- More extensive ammonia pretreatment. LB is treated with ammonia at elevated temperature or with aqueous ammonia at elevated temperature (150-190oC) for a short time.
- Hot water treatment. LB is treated with 180-230oC water for a short time.
- Dilute acid pretreatment. LB is treated with dilute hydrochloric, sulfuric, or phosphoric acid at elevated temperature (140-215oC) for a short time.
- Dilute alkaline pretreatment. LB is treated by a combination of an alkaline agent (eg. sodium hydroxide) and mechanical action.
- Lime pretreatment. Aqueous lime slurry is sprayed onto the biomass, which is allowed to sit for a period of time. After treatment, the biomass is washed liberally with water and the lime is recycled via kiln.
- Organosolv pretreatment. LB is treated with an organic solvent at elevated temperature (100-250oC). Organic solvents can include alcohols and glycols (methanol, ethanol, ethylene glycol), carboxylic acids, ethers, ketones, and phenols. Catalysts such as hydrochloric or sulfuric acid can be included.
- Oxidative delignification. LB is treated with an oxidizing agent such as aqueous hydrogen peroxide, or ozone. Air or oxygen in water at 150-350oC also has been used.
Each of these pretreatments has advantages and disadvantages, including different degrees of separation and degradation of cellulose, hemicellulose and lignin, raw material and equipment cost, and feedstock flexibility. Several have been used commercially or are near commercialization.
Conversion to Sugars
Sugars are the feedstock for most fermentations and several chemical and thermal routes from biomass to renewable fuels and chemicals. Hence, for these processes, it is mandatory for the cellulose and hemicellulose in biomass to be converted to sugars. It is for this reason that we include conversion to sugars in this post on biomass pretreatment.
After mechanical preparation by methods described above, conversion of cellulose and hemicellulose to sugars can be accomplished by three processes, each of which is the focus of commercialization by one or more companies.
- Enzymatic hydrolysis. The process usually involves a cocktail of enzymes to convert the cellulose to glucose and hemicellulose to a mixture of 5- and 6-carbon sugars that is usually rich in xylose. Efficient utilization of biomass will require that fermentations can convert both cellulose and hemicellulose sugars to the desired fuel or chemical products.
- Supercritical water hydrolysis of biomass to sugars. This process involves rapid exposure of biomass to water heated past the critical point (374oC, 22.1 MPa). Under these conditions, hydrolysis occurs rapidly.
- Treatment of biomass with a strong acid, usually hydrochloric acid or sulfuric acid. The acid must be separated from the product sugars and recycled to minimize waste generation or converted to a disposable or usable form (such as calcium sulfate, or gypsum, from sulfuric acid).
Lignin comprises 20% or more of most biomass, so for biomass-to-fuels-and-chemicals processes that utilize only cellulose and hemicellulose, lignin represents a significant yield loss unless it can be sold or burned for energy. This also means that very efficient conversion of cellulose and hemicellulose to sugars is imperative if biomass is to be used efficiently in production of fuels or chemicals. Processes that employ high temperature and acid catalysts to convert cellulose and hemicellulose to sugars can result in significant side products, such as furfurals and levulinic acid (Biomass to fuels , see Section 7.2, page 4073). These side products, along with lignin decomposition products, can inhibit fermentation of the sugars to the desired products, so minimization of these products can be important to downstream processing (Pretreatment 1 , page 77, Section 3.1).
Step 6: The Payoff
In this post, we have tried to give the reader an overview of the biomass pretreatment landscape, including the association between pretreatment methods and subsequent processing to make biofuels and renewable chemicals. In the final post in this series, we will focus on technologies that are involved in this last step, which is the payoff step for the biomass-to-fuels-and-chemicals industry.

Leave a Reply