Guest Post by Dr. Robert Humphreys
 Much has been written about the potential to replace some or most of petroleum with biomass as a source of fuels and chemicals and the massive, reliable supply of biomass that will be needed to achieve this objective. Before we delve into this 2nd generation biomass feedstock challenge, it might be worthwhile to devote one post to some of the important chemical differences between petroleum and biomass. Indeed, much of the processing to convert biomass to biofuels is required to address the chemical differences between biomass and petroleum.
Much has been written about the potential to replace some or most of petroleum with biomass as a source of fuels and chemicals and the massive, reliable supply of biomass that will be needed to achieve this objective. Before we delve into this 2nd generation biomass feedstock challenge, it might be worthwhile to devote one post to some of the important chemical differences between petroleum and biomass. Indeed, much of the processing to convert biomass to biofuels is required to address the chemical differences between biomass and petroleum.
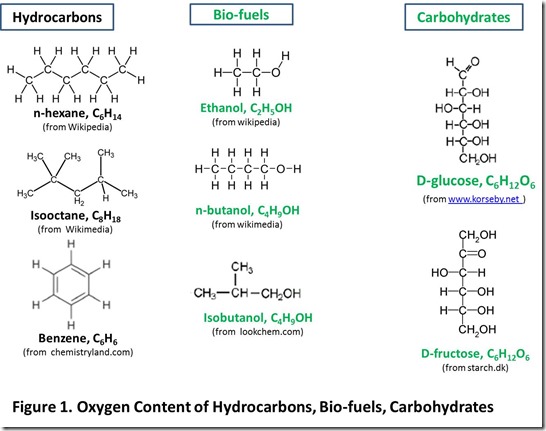
Petroleum is mostly hydrocarbons, or molecules composed of carbon and hydrogen. Biomass is mostly carbohydrates (ca. 70-80%, with the remainder mostly lignin), or molecules composed of carbon, hydrogen, and oxygen(see Note 2 below). For example, in Figure 1, we show the chemical structures of some important hydrocarbons produced from petroleum, several bio-fuels produced from biomass by fermentation, and several sugars (simple carbohydrates) produced from biomass. These structures illustrate a crucial difference between substances that are suitable for applications such as transportation fuels and carbohydrates: oxygen content. Substances that are suitable for transportation fuels contain a low level of oxygen or no oxygen.
Carbohydrates contain a high level of oxygen. High oxygen content reduces the number of hydrogen atoms directly attached to carbon atoms. For example, the carbohydrate glucose has the same number of carbon atoms and only two fewer hydrogen atoms than hexane (12 compared to 14), but 5 of the hydrogen atoms in glucose are attached to oxygen rather than carbon. Hexane contains no oxygen.
Oxygen content of a substance is directly related to the energy released during combustion. For example, combustion of hydrocarbons, butanol (iso-butanol or n-butanol), and ethanol releases more energy than combustion of carbohydrates or biomass. Published values for heats of combustion are listed in Table 1. These data help to explain why butanol (n- or iso-) is considered to be superior to ethanol as a biofuel target (butanol vs ethanol), as well as why hydrocarbons can be used to produce energy-dense fuels such as gasoline and diesel.
Table 1. Heats of Combustion Data
|
Substance |
Heat of Combustion, kJ/g (reference) |
|
n-hexane |
44.8 (Heat_of_combustion) |
|
ethanol |
28.9 (Heat_of_combustion) |
|
n-butanol |
36.2 (nButanol heat of comb) |
|
Iso-butanol |
36.0 (ibutanol heat of comb) |
|
glucose |
15.6 (Glucose heat of comb) |
|
dry wood |
18.9 (wood heat of comb) |
|
switchgrass |
18.1 (biomass combustion) |
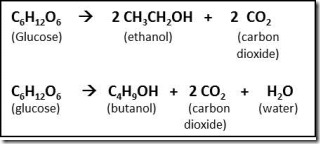
Data in Table 1 also show why removal of oxygen by substituting it with hydrogen is the principle focus of the biochemical and chemical processing to convert carbohydrates and biomass to biofuels2. When carbohydrates or biomass are converted to butanol or ethanol, most of the oxygen is lost as carbon dioxide during the process. For example, chemical equations for conversion of glucose to ethanol and iso-butanol are shown below:
The equations show that two carbons and about half of the glucose (by weight) are lost as carbon dioxide during fermentation of glucose to ethanol or butanol. Conversions are similar for biomass thermal processing to biofuels. This loss of carbon dioxide is required to supply the hydrogen needed to produce biofuels with much higher carbon-hydrogen bond content than the carbohydrate or biomass feedstock (see Note 3 below). In short, these equations show help to explain the low efficiency of carbohydrate (and biomass) conversion to biofuels (41% for iso-butanol, 51% for ethanol).
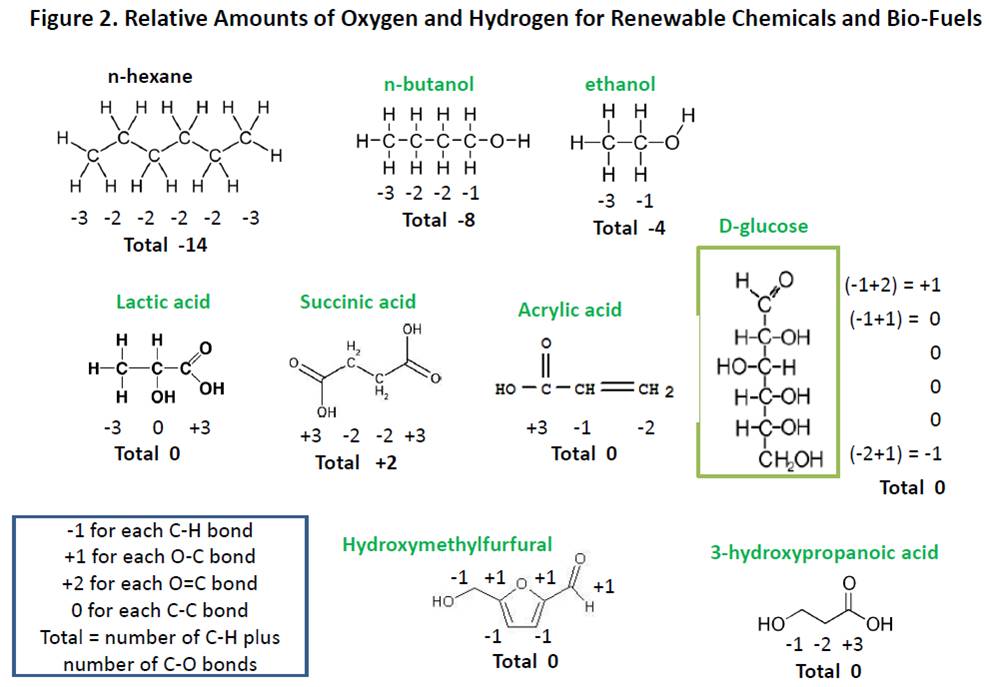
Biomass oxygen content is not an issue for production of many valuable, bio-based chemicals, including succinic acid, lactic acid (and PLA polymer), furfural and hydroxymethylfurfural (and related products, such as furan-2,5-dicarboxylic acid), acrylic acid, and 3-hydroxypropanoic acid (the fermentation product used to make bio-acrylic acid) because these molecules have a higher oxygen content and/or a lower content of carbon-hydrogen bonds than typical biofuels. Chemists would explain that renewable chemicals such as lactic acid, succinic acid and the others mentioned above are in the same or higher relative oxidation state than carbohydrates such as glucose. For this reason, it is not necessary to “free up” hydrogen by sacrificing biomass as carbon dioxide, as required to produce biofuels, which results in more efficient utilization of biomass in producing these renewable chemicals.
We can employ a simple system for comparing relative oxidation states of molecules to demonstrate equivalence or near equivalence of oxidation state of renewable chemicals and a typical carbohydrate like glucose. We assign a (-1) for each hydrogen atom attached directly to a carbon atom and a (+1) for each oxygen bond to a carbon atom. For carbon-carbon bonds, we assign a (0). We have done this in Figure 2 for glucose and the chemicals listed in the previous paragraph, along with the petrochemical hexane for comparison. It is easy to see that renewable chemicals such lactic acid and hydroxymethylfurfural contain the same relative amount of oxygen as glucose, while bio-fuels such as ethanol and butanol contain much less oxygen. Some of the conversions of glucose to the renewable molecules in Figure 2 rely on fermentation technology while others employ chemical technology to produce the target molecules, yet all of the conversions have the potential to be very biomass efficient since products are in the same relative amounts of hydrogen and oxygen directly attached to carbon as feedstock.
Biomass oxygen content is a major issue for economical production of biofuels and low-oxygen-content bio-based chemicals (biofuels digest oxygen content). This helps to explain why bio-based chemicals such as lactic acid (and PLA), succinic acid, and acrylic acid are receiving so much attention from major chemical companies (basf bioacrylic , basf succinic, dow bioacrylic , natureworks lactic ).
In the next post, we will examine lignocellulosic biomass agriculture, the first step in developing a reliable supply of biomass that will be so necessary for long-term success of biofuels and renewable chemicals.
Notes:
2) Petroleum and biomass are complex materials. Petroleum usually contains nitrogen, sulfur, and trace metals which need to be removed during processing. In addition to carbohydrates, biomass usually contains significant amounts of lignin and water and lesser amounts of protein, other biomolecules, trace metals and even silica.
3) We note that other properties are also important for a substance to be an acceptable for transportation fuel applications, such as the requirement for a liquid (carbohydrates and biomass are almost always solids), acceptable volatility, miscibility with other fuel components and acceptable performance when mixed with, or substituted for, other fuel components. Butanol is superior to ethanol in most of these properties, too.

xamine lignocellulosic biomass agriculture, the very first step is to develop a reliable supply of biomass that will be so necessary for long-term success of biofuels and renewable chemicals. This is very necessary
https://www.hplpolymers.com
Thanks to the excellent guide