Guest Post by Dr. Robert Humphreys
One can imagine two very different options for developing renewable versions of polystyrene (PS) and polyethylene terephthalate (PET):
- Develop “drop-in replacement” renewable versions of PS and PET that can be substituted directly for petroleum-based PS and PET with minimal effort and little or no increase in cost to end users of these polymers.
- Develop a renewable polymer (or polymers) with properties and cost very similar to PS or PET that can be used in most or all of the applications for these polymers. A renewable polymer with better properties or lower cost than PS or PET will have a better chance to replace PS or PET in existing or new applications.
These options apply to renewable versions of any high-volume, petroleum-based polymer. Commodity polymers are used by a very broad range of customers, each of whom has invested substantial effort (and, therefore, $) in developing processes and products based on petroleum-derived polymers with well-understood, reproducible properties and performance. Consequently, there is a built-in reluctance (call it a systemic reluctance) to replace existing, petroleum-based polymers with renewable alternatives unless renewable versions are true drop-in replacements or there is a significant economic incentive to switch. Of course, any new polymers, whether petroleum-based or renewable, face this same challenge. This should not be a surprise to anyone familiar with the market dynamics of the polymer business.
Refinery Flexibility and Market Demand
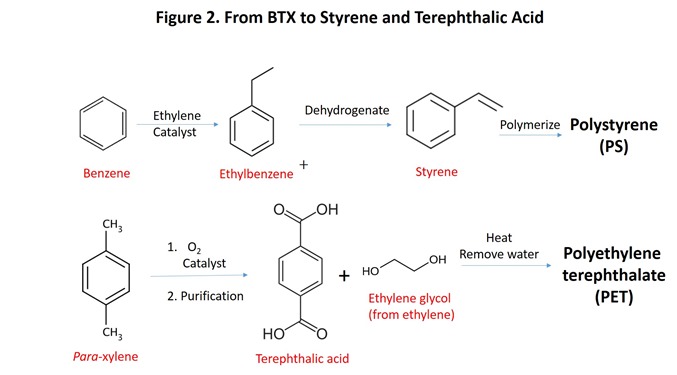
Modern US oil refineries produce massive amounts of BTX, the key raw material source for PS and PET, using “catalytic reforming” technology (see Figure 3, previous post in this series). Benzene, toluene, and xylene can be interconverted, allowing the refiner to respond to changes in demand for each product (Kirk-Othmer Encyclopedia of Chemical Technology, 5th Ed, Wiley-Interscience, 2004, Volume 18, page 565). Such flexibility required additional refinery investment but could be justified by allowing the refiner to respond with the most valuable product mix during a given market cycle. This is but one example of the efficiency of modern refineries that must be addressed if viable, renewable sources of BTX are to be developed. Without renewable BTX, there can be no renewable PS and PET.
Biomass as a Feedstock
Much has been written about biomass as the preferred choice for replacement of petroleum-based fuels and petrochemicals (see, for example, the Billion Ton Update 2016, BT Update 2016 ). Many of the challenges inherent in biomass as a raw material have been discussed in detail elsewhere (see Introduction to Biopolymers and Bioplastics, Part 3, https://polymerinnovationblog.com/). However, for anyone interested in renewable PS and PET, a few points about biomass are critical to feasibility of any initiative to replace petrochemical sources of BTX for the two most important PS and PET monomers (styrene and terephthalic acid, respectively):
- Petroleum is rich in hydrogen compared to biomass. BTX production from petroleum generates hydrogen as a co-product that is used in many other industrial applications, including other chemical manufacturing. Biomass conversion to BTX actually requires hydrogen, either from external sources (e.g. petroleum, natural gas) or by sacrificing some biomass to generate hydrogen (see Introduction to Biopolymers and Bioplastics, Page 38, https://polymerinnovationblog.com/), which can result in additional cost or reduction in raw material efficiency.
- BTX is one of a large number of product streams from modern refineries found in the USA. Although significant investment is required to separate and purify benzene, toluene, and the three xylene isomers, much of the investment to make BTX can be spread over a much larger fuels businesses. It is unclear if biomass will offer similar economic benefits for renewable BTX production.
- The world is awash in petroleum and natural gas and is likely to remain so well into the future. Low petroleum and natural gas prices put additional economic pressure on any renewable fuels and polymers initiative, sometimes in unpredictable ways (e.g. shale gas ethane conversion to ethylene increasing the global ethylene supply). Low oil and gas prices do not help investment in alternative energy and chemical sources.
Readers who have scaled-up and commercialized technology will understand that the process is fraught with issues that can arise from unanticipated technical problems that usually accompany scale-up, not to mention unexpected and untimely changes in the market, government regulations, customer preferences, competitive technologies, and global economics that can occur during development and commercialization. With this in mind, any attempt to predict which technology for renewable PS and PET might be commercially successful seems risky. When coupled with the paltry amount of detailed technical information that is typically available to assess actual performance early stage renewable technologies, such predictions seem positively futile. So, where does one go from here?
A Simple Approach
All technologies targeting replacement of petrochemical PS and PET with bio-sourced equivalents face many of the same challenges. For example, a large, reliable, efficient biomass supply chain is mandatory and will be complex enough that it won’t happen rapidly. The vagaries of biomass composition, even for a single feedstock type (e.g. switch grass, loblolly pine, corn stover, etc.) are challenging for all renewable technologies. Little precedent is available for many of the scale-up issues faced by most novel renewable technologies.
What about potential chemical/process efficiencies and process simplicity as a possible differentiators? In the next two posts, we will examine what we know about chemical/process efficiencies and process simplicity as a way to differentiate among technologies for replacing petrochemical PS and PET with biomass-derived equivalent materials. Our analysis will examine technologies in the following groups:
- Technologies involving bio-BTX.
- Alternatives to bio-BTX.
*Images taken from:
– Styrene, ethylbenzene, terephthalic acid, para-xylene, ethylene glycol images from Wiki images, Wikipedia, Wiktionary images


Leave a Reply