Guest Post by Dr. Robert Humphreys
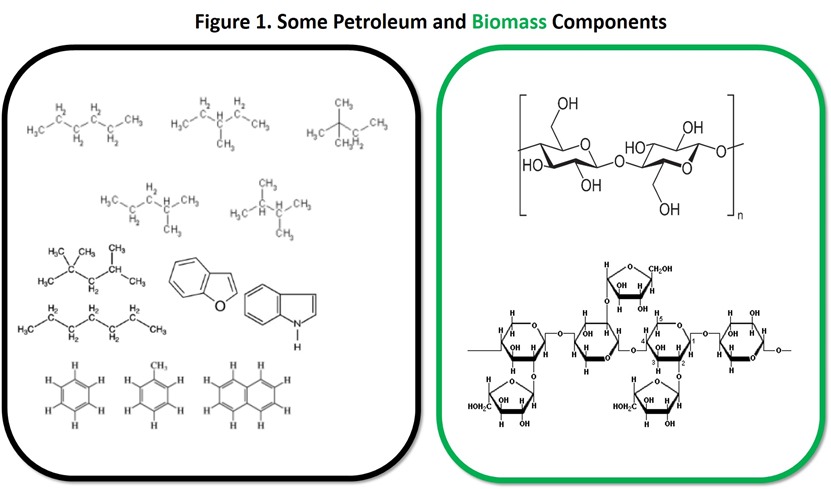
As explained in previous posts, most plant biomass (about 80% by weight) consists of carbohydrates (cellulose and hemicellulose), biopolymers that are rich in oxygen. In contrast, petroleum is comparatively rich in hydrogen and contains very little oxygen (usually less than 1% by weight, often much less). Figure 1 shows the structure of the major biomass components and typical components of crude oil so the reader can see the difference. On this basis, it should make sense that biomass may be most suitable as a raw material for production of chemicals and polymers that are relatively rich in oxygen while petroleum may be most suitable for chemicals and polymers that are relatively rich in hydrogen and low in oxygen. With this concept in mind, we will now examine a route from biomass to a renewable alternative to polyethylene terephthalate (PET) that benefits from the composition of biomass.
From Fructose to Renewable PET Replacements
The sugar fructose, the main component of high-fructose corn syrup, is important commercially because it is sweeter than sucrose (“table sugar”), a property that has been of significant value to the food industry. On the other hand, fructose has been of little interest industrially until recently, when the conversion of fructose to a potentially valuable chemical became relevant to the migration from petroleum-based to renewable plastics.
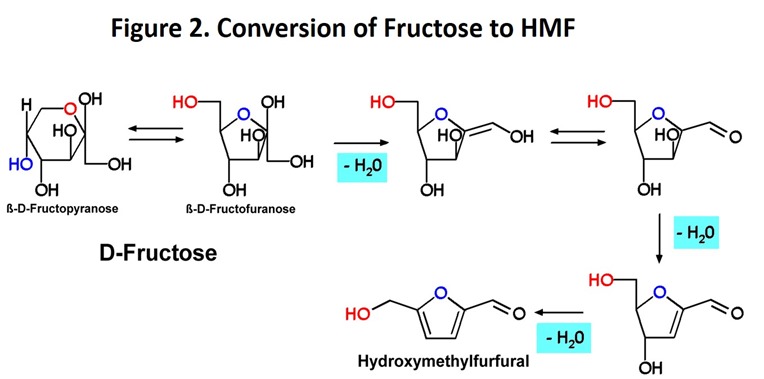
Conversion of 6-carbon sugars such as glucose and fructose to hydroxymethylfurfural has been known since the late 1800’s (see paragraph 3 in US Patent 3118912). The transformation is actually a rearrangement of the sugar molecule during which three molecules of water are lost and a 5-membered, oxygen-containing ring (a “furan” ring) is formed. For readers with some interest in organic chemistry, a simplified mechanism for this interesting reaction is shown in Figure 2 (image from Wikimedia, Fructose to HMF) using fructose as the sugar. The reaction requires an acid catalyst to proceed and research has demonstrated that fructose can be converted to HMF efficiently under practical conditions (see sugars to furans, pages 3-6).
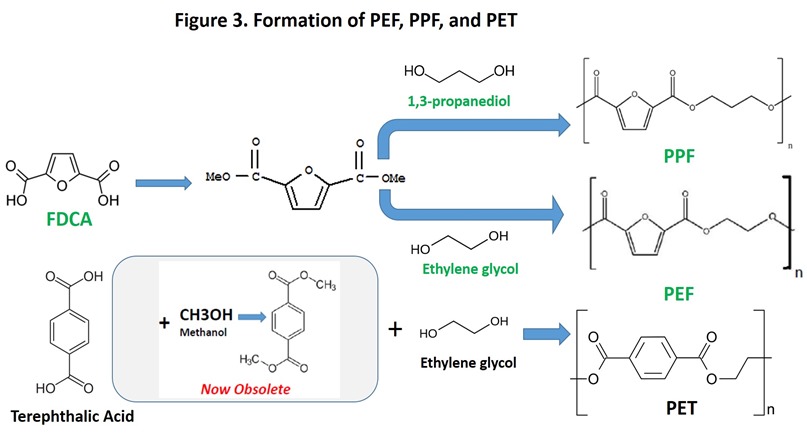
Conversion of sugars to HMF might have been less exciting to the chemical industry were it not for the facile conversion of HMF to furan-2,5-dicarboxylic acid (FDCA, see sugars to furans, page 9), a molecule with properties that make it a potential substitute for terephthalic acid in PET. The substitution results in a new thermoplastic, known as poly(ethylene furan-2,5-dicarboxylate), or PEF. PEF is very similar to PET in many of its properties, but may have significantly better gas barrier properties. Better gas barrier properties may create a strong pull for PEF from beverage and food packaging markets, especially if 100% renewable PEF can be delivered cost effectively. Several recent developments have generated considerable excitement about the potential of PEF to become a commercial reality, including:
- Announcement of a JV focused on FDCA and PEF between a company based in renewable technologies and one of the world’s leading chemical manufacturers (Avantium and BASF).
- Announcement of a breakthrough in producing FDCA (DuPont and ADM), which also opens the possibility of poly(propylene furan-2,5-dicarboxylate), or PPF, a 100% renewable polymer that also is potential PET replacement. See Figure 3 for structures of PEF and PPF compared to PET.
- Availability of fructose. Major food and beverage companies have been switching from high-fructose corn syrup (HFCS) to alternative sweeteners because of a perceived connection between the HFCS and obesity. Consequently, fructose capacity is available. HFCS is produced by enzymatic isomerization (i.e. rearrangement) of starch-based glucose to a very concentrated fructose solution (77% sugars that are 90% fructose), which is a potential feedstock for HMF production.
Apparently, the preferred route to PEF involves the dimethyl or diethyl ester of FDCA, analogous to the now obsolete method for manufacturing PET from dimethyl terephthalate (Figure 3). Extra steps mean extra cost, so presumably the direct polymerization of FDCA with ethylene glycol will receive a lot of attention. On the other hand, low-cost sugar sources, including cellulosic sugars, could make PEF economically attractive. Based on recent announcements mentioned above, we may find out soon.
Taking Advantage of Nature’s Work
Elegant, as in “pleasingly ingenious and simple”, is not a term that should be used to describe the vision to replace petroleum with biomass as a source of fuels and chemicals, as a few minutes examining the DOE 2016 Billion Ton Report will confirm. The transformation is so complex that any prediction of the eventual outcome is of little value. In stark contrast, making HMF and FDCA is conceptually simple:
- HFCS is manufactured from cornstarch (or, potentially, any other source of glucose, such as cellulose) on a scale large enough to supply a significant portion of the market for PET packaging until low-cost cellulosic glucose is available (see HFCS production USA);
- Conversion of fructose to HMF has been known for over a century and efficient conversion was reported before recent commercialization efforts began. Process improvements can be expected during commercialization;
- HMF is in the same relative oxidation state as fructose, taking advantage of the chemistry Nature offers. No hydrogen is needed for the transformation; this trumps biomass-to-BTX routes to bio-terephthalic acid, which require added hydrogen that must be removed in the next step when para-xylene is oxidized (see the previous post in this series, August 22, 2016);
- HMF can be converted to FDCA very efficiently by catalytic oxidation with oxygen;
- Commercial sources of renewable ethylene and 1,3-propylene glycols necessary to manufacture PEF and PTF are available;
- PEF may offer improved properties to customers, providing a much needed market driver for this material beyond renewability;
- Finally, major chemical companies with long track records for commercialization of polymers and chemicals are committing resources to make FDCA-based polyesters a reality.
In our eBook, Introduction to Biopolymers and Bioplastics, Jeff and I devoted a section to the many renewable polymers that have been a part of industrial technology for decades and, in some cases, centuries. Examples include starch derivatives, cellulosic polymers, alkyd resins, and polymers made from pine chemicals, as well as fibers such as cotton and rayon, that are still indispensable ingredients in many consumer and industrial products. These materials are commercially successful because they offer the performance customers need at a price they are willing to pay. Renewability is a bonus but performance at the “right” price is why customers pay for them. Polyesters derived from HMF may succeed for the same reason.
Image Acknowledgements
Molecular structures and other images were taken from:
– Figure 1: Hexanes image, Benzene, naphthalene, anthracene images, Isooctane images, Cellulose image, Hemicellulose image
– Figure 3: Dimethyl_terephthalate, Terephthalic acid image, Furandicarboxylic acid image, Furandicarboxylic acid dimethyl ester image, Propanediol image, Ethyleneglycol image, PPF image, PET image

Leave a Reply