Guest Post by Dr. Robert Humphreys
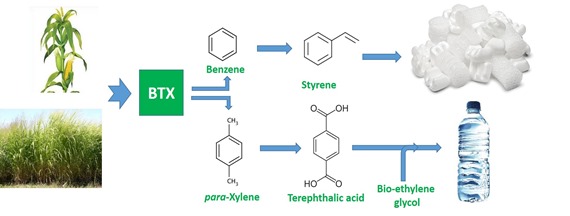
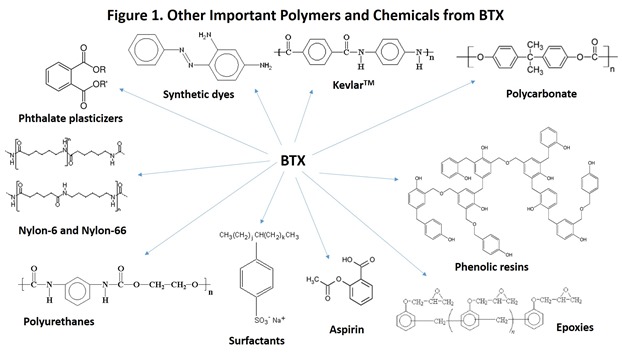
This post will focus on technologies for conversion of biomass to renewable BTX as a source of renewable polystyrene (PS) and polyethylene terephthalate (PET), as well as many other valuable polymers and chemicals that are so much a part of life in the modern world (Figure 1). Many initiatives have focused on developing renewable BTX, mostly based on a perceived need to replace petroleum as the source of the ubiquitous food and beverage packaging plastic, PET. We will describe several routes to bio-BTX as a source for renewable PS and PET and also a key challenge facing these technologies.
Conversion of Biomass to BTX Requires Hydrogen
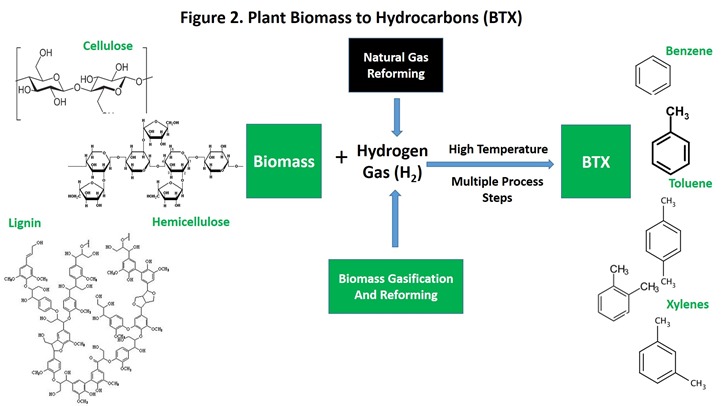
Agricultural biomass is a product of photosynthesis in which plants use light energy to “split” water, providing hydrogen to convert carbon dioxide (CO2) from the atmosphere into the 6-carbon sugar known as glucose, the raw material for biosynthesis of the three main components of plant biomass: cellulose, hemicellulose, and lignin. In “chemist speak”, plants use hydrogen from water to reduce (= add hydrogen to and remove oxygen from) CO2 to build glucose. However, replacement of oxygen with hydrogen in glucose and biomass is far from complete. The major components of BTX, namely benzene, toluene, and xylenes, are hydrocarbons, which are substances that contain only carbon and hydrogen (i.e. no oxygen), while sugars, cellulose, hemicellulose, and lignin still contain substantial oxygen. Thus, conversion of plant biomass to hydrocarbons like BTX requires further substitution of hydrogen for oxygen. A simple illustration of the overall process can be found in Figure 2.
So how can plant biomass be converted to hydrocarbons such as BTX in a process that is both practical and scalable? Most important, where will the hydrogen come from? We will look at two different approaches in the remainder of this post: biomass pyrolysis; and fermentation of plant-derived sugars.
Biomass Pyrolysis
Heating of biomass to high temperature (typically around 6000C), known as pyrolysis, breaks the large biomass molecules into smaller molecules. In this sense, pyrolysis is analogous to petroleum cracking that is the first step in crude oil refining. Pyrolysis is conducted in reactors designed to exclude oxygen and to minimize the time the biomass spends at high temperature (usually under a second, known as residence time). A number of pyrolysis processes capable of producing BTX are in different stages of “commercial development.” The various processes differ in a number of important ways that cannot be covered in a short blog post (readers interested in more detail can refer to a number of publications, for example chapter 8 in Catalytic Process Development for Renewable Materials or Introduction to Biopolymers and Bioplastics, pages 51-60). Nevertheless, each process requires additional hydrogen to remove oxygen from the biomass raw material. So, where will this hydrogen come from?
Hydrogen gas (H2) is a critical industrial raw material used in immense quantities in globally critical industries such as fertilizer production and removal of sulfur and nitrogen (air pollutants) during crude oil refining. Hydrogen gas is by far the most common substance in the known universe but is not found in practical amounts on earth (0.00005% in air). Consequently, H2 must be manufactured by an economical process. This is accomplished primarily by steam reforming of natural gas, a raw material that is abundant and cheap.
Close examination of Figure 2 will show that added H2 can also come from biomass. Gasification followed by steam reforming to produce H2 has been demonstrated (for a review, see Huber et al, page 52-56). However, the economics of this H2 source will need to be demonstrated in a world where natural gas abundance seems like the norm.
Biomass to BTX via Fermentation
Direct microbial conversion of biomass or biomass-derived sugars to BTX is unknown. Simply put, microorganisms are not equipped to produce simple aromatic hydrocarbons such as benzene, toluene, and xylene. Consequently, almost all of the effort to date has focused on microbial fermentation of biomass to a product that can be converted to para-xylene (pX), one of the three xylene isomers. The reason for this is market-based: pX is the raw material for production of terephthalic acid (TA), one of two building blocks for the very important commodity polymer PET (see opening graphic). Adverse publicity about the colossal quantities of plastic food and beverage packaging waste has resulted in a perceived need for a renewable version (or replacement) of PET, particularly among major, global food and beverage companies.
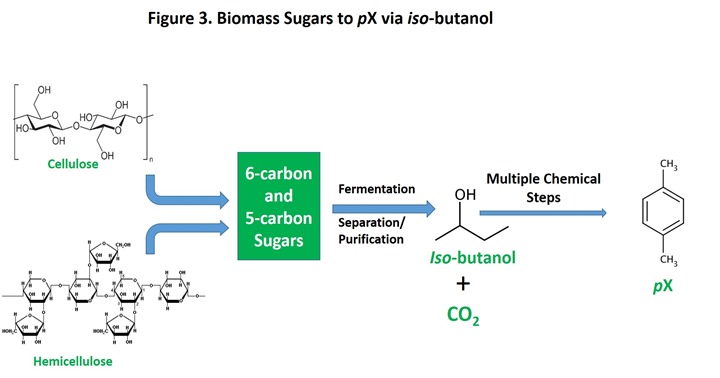
Practical processes for conversion of fermentation products to pX are limited. Iso-butanol is the option that has received the most attention. The overall process is shown in Figure 3.
An engineered version of E. coli converts glucose to iso-butanol, followed by multiple chemical steps that convert iso-butanol to pX. As in the pyrolysis route, the hydrogen necessary to reduce the oxygen in biomass (glucose in this process) must come from somewhere and in this case, it is supplied during the fermentation by converting some biomass to CO2, providing the required hydrogen in the process. For this reason, the yield of iso-butanol is under 50% by weight, meaning that over half of the glucose is sacrificed as waste CO2. This, then, is the upper limit on the overall yield of biomass to pX for this route.
Commercially successful, commodity chemical processes that convert less than half of the raw material to salable products are hard to find.
Prognosis for Commercial PS and PET based on bio-BTX
There are many challenges in developing biomass as an economical source of BTX and, ultimately, renewable PS and PET. This post has highlighted what we believe is the most significant one, namely necessity for a hydrogen source. For biomass pyrolysis, the only practical source at present is natural gas, which effectively means producing greenhouse gas to make renewable polymers. As for microbial fermentation and chemical conversion as a source of pX, the yield based on biomass seems like a non-starter. Finally, readers may have noticed that production of renewable PET requires renewable TA, a building block that has a high oxygen content, effectively wasting the hydrogen used to generate pX from biomass!
In the next, and final, post in this series, we will examine a renewable version of a polymer that might replace PET as an example of an approach that benefits from the oxygen content of biomass rather than treating it as a problem.
*Figure Acknowledgement
Molecular structures and other figures taken from:

Dear,
The writer seems to doubt the feasability of a succesful bio-based plastics industry. Recently, a major beverage producing company invested in a bio-based PET- bottle production facility in Madison, USA. (https://www.ptonline.com/blog/post/coca-cola-debuts-first-100-biobased-pet-bottle). The same company also invests in a commercial plant in the Netherlands (https://www.packaging-gateway.com/projects/-coca-cola-plant-based-bottle/) for the production of 100% bio-based PET-bottles. This seems to indicate the feasability of such materials. Is the writer’s scepsis based on the current economical climate, or rather on fundamental chemistry considerations such as yields and purity of processes?
Sincerely,
Pieterjan.
Hydrogen is not a necessity in the Anellotech process to make BTX mixtures. This process operates on a carbon-rejection principle, and is in some ways analogous to refinery fluid catalytic cracking, but unlike FCC does not utilize a riser-reactor. Thermal pyrolysis of lignocellulosic biomass results in formation of oxygenated intermediates. These are converted over HZSM-5 zeolite catalyst in the vapor phase into BTX and side products including char and coke on catalyst (e.g. the carbon rejection pathway). Oxygen is rejected as water, CO and CO2. Formation of coke & char is important for the process energy balance, because their combustion in a separate catalyst regenerator vessel provides the energy to drive the pyrolysis and the catalytic reactions going on in the reactor (this is the analogy to FCC). Continuous catalyst circulation transfers regenerated catalyst to the reactor and spent catalyst to the regenerator. The renewable BTX mixture can be separated and upgraded by typical refinery- or steam-cracker based aromatic separation and purification facilities.
To Pieterjan,
First of all, thank you for the comments. I’m sorry it took so long to reply, but life has been busy and I did not look for comments until recently.
The issue is economics when I use the term “feasibility”. There is no question that PET can be made from biomass by multiple chemical or biochemical/chemical routes; a colleague of mine and I wrote a book chapter several years ago that reviews routes to renewable aromatics that included BTX, terephthalic acid, and PET. However, synthetic feasibility is no guarantee of economic feasibility, which is what matters in commercialization (i.e. renewable PET at a cost that is competitive with petroleum-based PET).
“Drop-in equivalent” renewable versions of petrochemically derived plastics face monumental challenges before successful (= profitable, with or without subsidies) commercialization. Consider cost. Since we now know that petroleum and natural gas are far more abundant and accessible at a market-acceptable cost than was thought a decade ago, we cannot expect the economics to change dramatically in the foreseeable future. I might add that innovation in petroleum exploration and production continues to reduce the cost of bringing on new reserves, which should keep petroleum cost well below what it was in the heyday of investment in renewable energy and renewable chemicals, assuming no major supply disruptions.
I have written at this site in the past about the steps and challenges involved in building a biomass supply chain of a scale that can serve even a few drop-in equivalent renewable versions of petrochemically derived plastics and biofuels. This is a monumental task that is of such a size and complexity that there is little chance it will happen predictably. Assuming that renewable chemicals get to share the cost with biomass-derived fuels will not simplify the challenge.
I might add that there is no evidence that the consumer will pay more for renewable versions of petroleum-based plastics. A small sector of consumers may be willing to pay a “green premium”, but there is no evidence (i.e. consumers paying at the checkout, not polls or surveys) that most consumers will pay more. The way the global economy is looking, I wouldn’t get my hopes up anytime soon.
This means that renewable plastics will need to compete on cost or offer improved properties that have enough advantage to justify a premium. One such plastic, PLA, has become mainstream enough that it needs to be considered in planning plastic recycling. There are others with similar potential. PEF is possibly even more straightforward than PLA for reasons I outlined in the subsequent post in this series. Two of the premier global chemical companies (chemical companies, not energy companies) are investing in PEF, presumably because the economics make sense and PEF offers improved barrier properties. Also, as with lactic acid, PEF technology benefits from what Nature does best, which is produce lots of biomass that is rich in oxygen. And there is plenty of raw material capacity available. If I was a betting person, this is where I would place my stack of chips.
Thanks again for the comments. I hope answered your question satisfactorily.
Bob Humphreys
To Csorensen:
I guess I need to clarify what I mean by hydrogen. Biomass (cellulose, hemicellulose, and lignin) is oxygen-rich, with cellulose (C6H10O5) and hemicellulose being richer in oxygen than lignin. All three are in a higher relative oxidation state than BTX, which means that production of BTX from biomass must be a reductive process. The reducing equivalents come from the biomass, so hydrogen gas is not required for the process, as you state. However, this means that the process must generate the reducing equivalents by converting some biomass into CO2, analogous to the way ethanol is produced during glycolysis through decarboxylation of pyruvate and reduction of the resulting acetaldehyde to ethanol by NADH.
I presume that most of the coke and char results from pyrolysis of the lignin, so lignin also may be a source of hydrogen. If this is correct, then this may be an advantage of your technology relative to other pyrolysis technologies.
All processes that convert structural biomass to reduced products, such as ethanol, BTX, and butanol must generate reducing equivalents by sacrificing some (sometimes even more than half) of the biomass raw material as CO2 (or coke and char) or must add some form of reducing equivalents (such as H2).
Thanks for taking the time to reply to our post.
Bob Humphreys