Guest Post by Dr. Robert (Bob) Humphreys
 A polymer or plastic must be biodegradable to be compostable. The reverse is not necessarily true, that is, a polymer might be biodegradable without being compostable. While this may seem inconsistent, biodegradation can occur under a variety of conditions and over a long time frame (up to years or even decades), possibly assisted by environmental factors such as photodegradation from exposure to UV radiation from the sun or physical degradation through abrasion (1). Biodegradation during composting must occur in a much more limited time frame, typically within a period that makes backyard or industrial composting practical; this constraint seriously limits the chemical structures that allow a polymer to biodegrade in a composting environment. In this post, we provide an overview of what is known about polymer compostability since it is one of the alternatives to landfills for waste plastic disposal. As always, reliable determination of any material property requires scientifically valid test methodology, so this is where we begin this post (for a very detailed discussion of polymer composting, see Compostable Polymer Materials).
A polymer or plastic must be biodegradable to be compostable. The reverse is not necessarily true, that is, a polymer might be biodegradable without being compostable. While this may seem inconsistent, biodegradation can occur under a variety of conditions and over a long time frame (up to years or even decades), possibly assisted by environmental factors such as photodegradation from exposure to UV radiation from the sun or physical degradation through abrasion (1). Biodegradation during composting must occur in a much more limited time frame, typically within a period that makes backyard or industrial composting practical; this constraint seriously limits the chemical structures that allow a polymer to biodegrade in a composting environment. In this post, we provide an overview of what is known about polymer compostability since it is one of the alternatives to landfills for waste plastic disposal. As always, reliable determination of any material property requires scientifically valid test methodology, so this is where we begin this post (for a very detailed discussion of polymer composting, see Compostable Polymer Materials).
Standard Test Methodology for Biodegradability in Composting
A valid claim of polymer compostability must be supported by well-defined test methodology performed by a third-party testing organization that is certified to perform such testing (2). This type of constraint is vital for all testing that involves serious potential economic, safety, environmental, or health risk. In the USA, the accepted test method is ASTM D5338-98 (see ASTM D5338-98), with specifications for “compostable” defined by ASTM D-6400-04 (see ASTM D6400-04). In parts of the USA that have regulations (e.g. California), only polymers and plastics that meet these standards can be labelled as compostable. Relevant European and international standards for plastic compostability are also listed in ASTM 6400.
Compost test methodology should be designed to mimic “real” world conditions in a controlled environment that is required to provide comparable data for different plastic materials. The test conditions also can provide some indication of expected degradation of a given plastic (or polymer) in a particular composting environment where conditions might be different and less controlled. For example, a backyard compost heap probably will not achieve the composting rate of a modern industrial facility that is designed to achieve optimal composting conditions.
The ASTM 5338 procedure involves incubation of the plastic or polymer sample in fresh compost (the “inoculum”) obtained from biologically active compost. The apparatus setup allows temperature and humidity control (580C, or 1360F; saturated humidity) and continuous flushing with air. Biodegradation is measured by carbon dioxide gas evolution. Inoculum activity is indicated by subjecting cellulose, a known, compostable standard, to the same compost as a control. ASTM 6400 requires 60% of the theoretical organic carbon to be converted to CO2 in 180 days for a single polymer or for each component in polymer blends and 90% for formulated polymers such as plastics. Most compostability testing also requires additional analyses to limit impurities such as heavy metals, PCBs, and agents that are aquatically toxic (see Compostable Polymer Materials, page 94); since the ultimate fate of compost is in soil remediation and agricultural applications, the reason for these requirements should be clear.
Structural Features of Polymers That Are Compostable
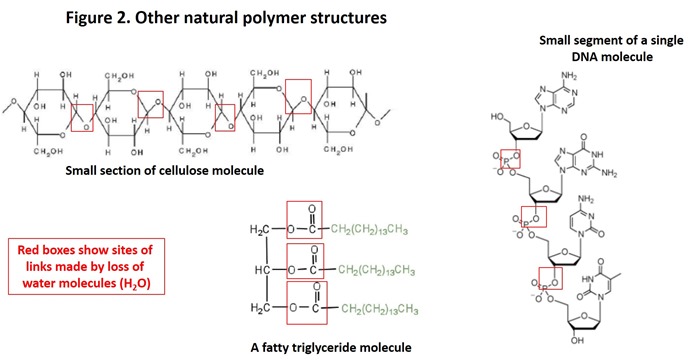
Most natural polymers can be categorized as carbohydrates (e.g. starch, cellulose, hemicellulose, chitin), proteins (e.g. gluten, casein, enzymes, collagen), nucleic acids (e.g. DNA, RNA) and lipids (e.g. fats) and are biodegraded easily in the environment and in compost (3). Lignin is a complex structural polymer found in plants and the second most abundant polymer in Nature; it is more slowly biodegraded and contributes significantly to soil organic carbon (4) that is a critical component of fertile soil.
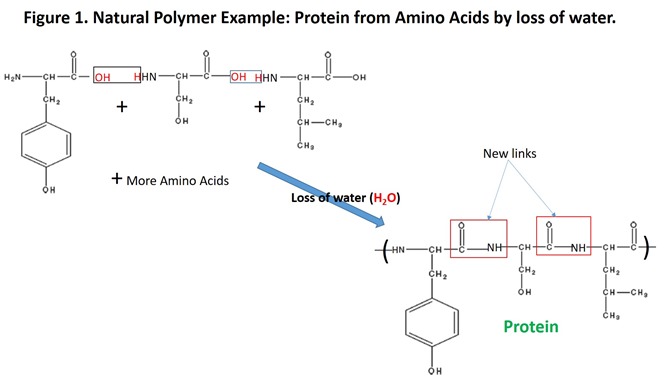
With the exception of lignin, construction of the natural polymers listed above is conceptually a simple process involving linking of small molecules together to form much larger molecules (similar to links in a chain); addition of each new link also “releases” a molecule of water (5). For example, proteins are constructed by linking amino acids together; carbohydrates by linking sugars together; nucleic acids by linking nucleotides together; fats by linking fatty acids to glycerol (see the Figures 1 and 2). The linking process involves special biological catalysts called enzymes which are necessary for living organisms to conduct the processes of life under conditions that are acceptable for life (generally from above freezing to below boiling of water, although exceptions are well known).
Nature is expert at linking these biopolymers together and also at “delinking” them (i.e. taking them apart). In the composting process, delinking of some easily-degraded biopolymers such as starch occurs as the composting temperature rises while more recalcitrant biopolymers such as cellulose require microorganisms such as fungi and certain bacteria, which excrete special enzymes that break the links to reform smaller building blocks such as amino acids and sugars. The smaller molecules are absorbed more readily by the microorganisms to be used as food. The key point here: what Nature creates Nature is quite capable of destroying.
The preamble above illustrates a critical point about biodegradable polymers, whether they are natural or man-made (i.e. synthetic). Over several billion years, Nature has become expert at making and breaking certain kinds of links in polymers. Man-made polymers that contain such links are more likely to biodegrade easily than those that don’t (e.g. under composting conditions). While this is not a steadfast rule, it is an excellent guide to designing biodegradable polymers.
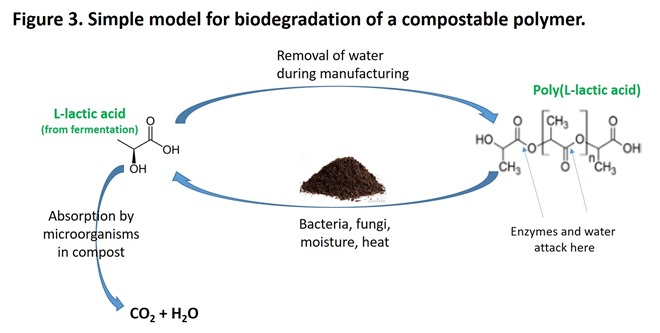
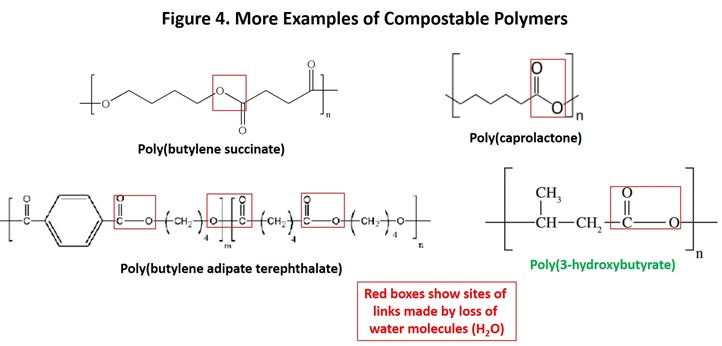
As Figures 3 and 4 demonstrate, most man-made compostable polymers contain links similar to those found in biopolymers. Also shown in Figure 4 is poly(3-hydroxybutyrate), a polyester that is produced by a bacterium as an energy storage material similar to the role of fats and starch more complex organisms. Note the similarity in structure between this polymer and others in the figure. Other compostable polymers are known, including modified starch and cellulose derivatives and polyamides, each of which contain links that are labile in active compost.
Finally, this post would not be complete without mentioning blends of compostable polymers, such as starch, with synthetic polymers that do not biodegrade. Such blends perform as expected: the starch or other biodegradable polymer erodes, leaving a matrix of synthetic polymer that does not biodegrade. It is doubtful that any benefit is offered by partial compostability.
In our next post in this series, we will examine some of the issues that make broad application of composting of appropriately biodegradable polymers an impractical option for polymer disposal in the current world of plastic packaging.
Additional References, Links, and Comments
1. Most plastics will show signs of degradation in the environment, depending on the type and extent of environmental exposure. Most of the evidence for degradation is based on physical test methods (e.g. microscopy, tensile strength, brittleness, visual appearance) and should not be confused with biodegradation testing. In some cases, such physical degradation may contribute to biodegradability, but not for most synthetic polymers and plastics. Some polymers photodegrade, some oxidize (many plastic formulations contain antioxidants), some erode through abrasion (e.g. plastic containers on a beach), some erode via wear (what is the fate of the tread rubber that wears from a tire during its useful life?). Evidence is accumulating to suggest that such plastics and polymers degrade to recalcitrant microparticles that accumulate in the environment, the consequences of which are far from being understood (e.g. Microplastics ref 1; Microplastics ref 2.
2. Lists of labs that are certified to perform composting tests that meet ASTM, EN, and ISO testing standards are available on the web. See, for example, List source 1 . A comprehensive list of polymer degradation test procedures can be found at Degradation test methods lists.
3. Fats, although very large molecules, are much smaller than most biopolymers but can be important components of biopolymers (e.g. lipoproteins), as can small carbohydrates (e.g. glycoproteins); fats biodegrade easily during composting (SOC1, page 6).
4. Soil organic carbon in compost is often referred to as humus, although the details of humus structure and how humus forms are unclear (see SOC1, SOC2).
5. We put “releases” in quotes because the actual biological process is much more complicated and a molecule of water may not be produced in the main link-forming process. Nevertheless, in the laboratory, a chemical reaction of the type shown in the figure does release water which usually is collected by condensation of steam; hence the name condensation reaction, the name given to all chemical reactions of this type.
Figure Acknowledgements
Figure 1:
Figure 2:
Figure 3:
1. Compost pile
2. PLA image
Figure 4:
1. Poly(butylene succinate) image
2. Poly(3-hydroxybutyrate) image

Leave a Reply