Guest Post by Dr. Robert (Bob) Humphreys
In the previous nine posts on plastic recycling and disposal, we focused on two approaches that require sorting: plastic composting; and separation of plastics that have commercial value in reuse. As we have seen, these methods do not provide a comprehensive solution to the waste plastic problem now and are unlikely to do so in the foreseeable future.
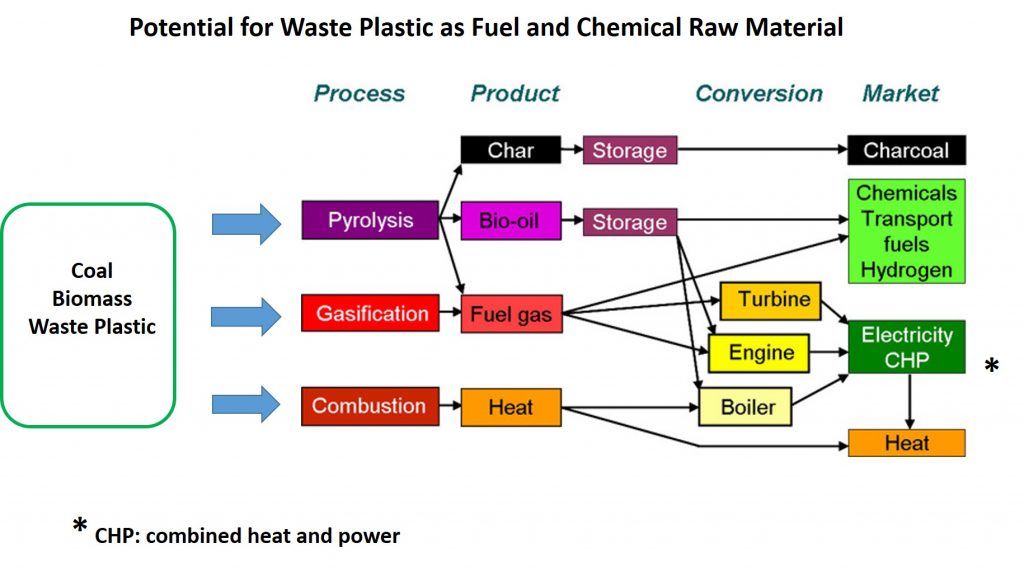
Landfilling municipal waste has fallen out of favor in most of the industrialized world, leaving incineration as the only obvious option for disposing of unrecycled waste plastic. Coupling heat recovery with incineration can improve the environmental impact of the process. Nevertheless, modern, clean incineration technology still converts the carbon in waste plastics to CO2, with the heat generated being of relatively low economic value, which seems like a wasteful way to dispose of a potentially valuable resource. So, one is prompted to ask if there are other options for capturing some of the potential value locked up in the chemical composition of plastics? This and the next several posts will describe one such option, namely thermal technologies for converting waste plastics, and municipal solid waste, into fuel and chemicals.
Thermal technologies: Old and New
Mankind has practiced thermal treatment technologies to produce fuel from biomass for centuries. For example, charcoal was produced by carefully controlled “burning” of wood while limiting the air supply (and, therefore the oxygen), usually by covering the wood pile with moist leaves and dirt (see figure below). Most of the hydrogen present in the wood was lost during the process as water vapor, methane, and hydrogen gases, leaving charcoal, composed almost entirely of carbon, and tars that collected at the bottom of the pile. Such charcoal was the principal fuel used to achieve the high temperatures required for iron smelting while the tar, particularly from coniferous wood, was useful for waterproofing wood in ship building.
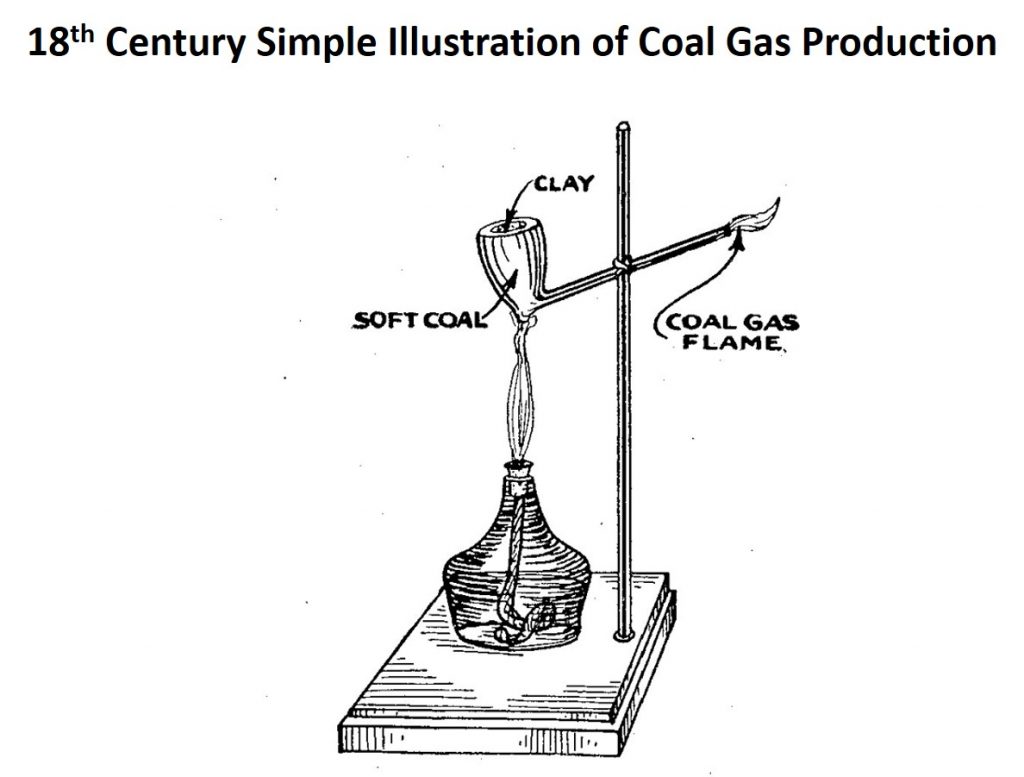
In the 19th century, coal gas was produced by heating coal to high temperature in the absence of air. This destructive heating produced a gas that contained a mixture of carbon monoxide, methane, hydrogen, and carbon dioxide that could be used as a source of both heating fuel and illumination, including street and household lighting (supplanted by electricity in the 20th century). Readers who have watched the BBC series “Sherlock Holmes” starring Jeremy Brett will have seen such street lighting in action in London.
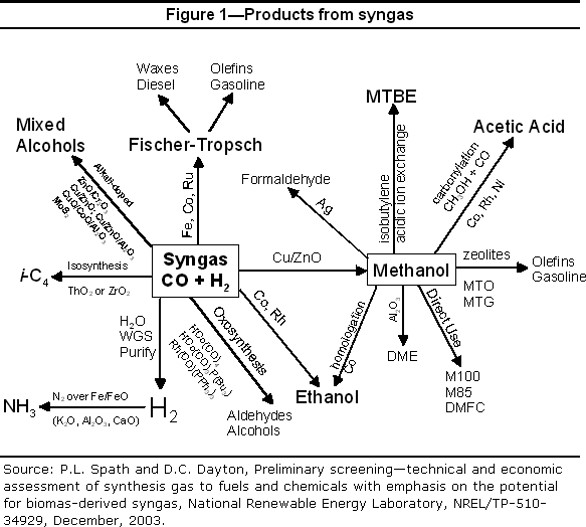
“Syngas” technology based on coal was developed in the 20th century, driven in large part by the lack of petroleum reserves in coal-rich Germany prior to WW2. Syngas technology converts coal to a mixture of carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2), with smaller amounts of other gases. Since coal is rich in carbon but low in hydrogen (compared to petroleum), additional technology, called the “water-gas shift reaction”, is used to generate additional H2 by reacting CO with water (H2O), converting CO to CO2. Fischer-Tropsch technology coupled with syngas provides an economically viable route to both diesel fuel and a broad range of important, basic organic chemicals, effectively decoupling transportation fuel and chemical production from petroleum. As we will see in the next post, this fact has been noted by the municipal waste recycling industry as a potential pathway for converting waste plastic to fuels and chemicals.
Finally, recent interest in using non-food biomass as a renewable alternative to petroleum for production of fuels and chemicals has resulted in pyrolysis technologies for converting biomass to “bio-oil” as well as basic chemicals (see page 58-59, “Introduction to Biopolymers and Bioplastics”, eBook available on this site). Several different pyrolysis technologies have been demonstrated, differing in temperature and heating time. Waste plastics have a number of potential advantages over biomass for conversion to bio-oil and chemicals, such as a much higher level of hydrogen attached directly to carbon than found in biomass. It should not be surprising, then, that thermal technologies for converting waste plastics to fuels and chemicals are being explored actively by the waste recycling industry.
Thermal technologies for waste plastic recycling
In previous posts in this series, we discussed the complexity of waste plastic recycling faced by local governments and companies that are responsible for municipal waste disposal. Waste plastic film is a particularly challenging problem since, unlike rigid plastic containers, many films are not single polymer materials but rather are multi-layer laminates of different plastics and even metals such as aluminum. The layers are very thin (measured in microns, µ, or millionths of a meter) and are strongly bonded, making separation almost impossible. Most waste film currently is destined for either landfills or incineration. However, high polyolefin content makes waste plastic film attractive as a potential raw material for thermal conversion to fuels and chemicals by methods briefly mentioned above. The next post will describe some of the effort to develop these technologies.



Leave a Reply