 In the previous post we reviewed the concept of vitrification during thermoset curing. In this post we will give some examples of how the cure temperature can impact the degree of cure and the glass transition temperature during processing.
In the previous post we reviewed the concept of vitrification during thermoset curing. In this post we will give some examples of how the cure temperature can impact the degree of cure and the glass transition temperature during processing.
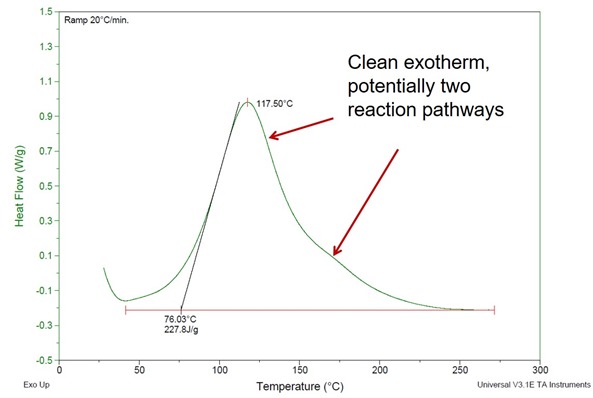
In the first case, we will consider the curing of a two part epoxy-amine system at 80oC. If the goal was to get a Tg as high as possible, would this cure temperature work? In order to investigate the cure process, a DSC scan was run on the uncured mixture. In Figure 1, the heat flow is plotted as a function of temperature for a 20oC/min ramp to over 250oC. This is typically done to get an indication of the cure onset temperature, the temperature at the maximum heat flow, and the shape of the exotherm.
Figure 1. Heat flow as a function of temperature for a two part epoxy amine system.
In the DSC in Figure 1, the onset temperature is 76oC. The onset is usually determined as shown in Figure 1 the the intersection of the baseline and a tangent to the slope of the initial heat flow. The good news is that at 80oC, the reaction will start. Inspection of the initial part of the DSC curve shows the reaction starts slightly lower than 50oC. There is one main peak in the exotherm and the shoulder after the peak maximum could indicate potentially two different reaction pathways. Without going into too much chemistry, the main peak could be the nucleophilic amine opening the epoxy ring. The higher temperature reaction may be the secondary hydroxyl (formed during the epoxy ring opening reaction) reacting with another epoxy ring. After the initial DSC scan, the DSC cell was cooled back to room temperature and a second scan at 20oC/min was performed.
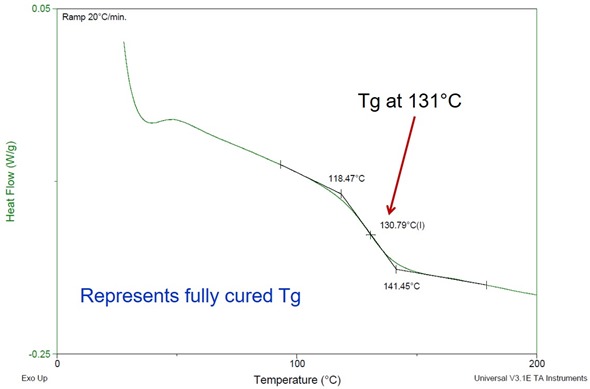
Figure 2. Heat flow as a function of temperature for a two part epoxy amine system during a second scan.
One should note that during the initial DSC scan, the temperature was raised well above the peak reaction rate to ensure the epoxy-amine system fully cured in the DSC pan. The second scan shows a Tg of 131oC. Note that the fully cured Tg is well above the cure temperature. In our discussion about vitrification we noted that the reaction rate decreases significantly with the Tg = Tcure. Note the reaction rate does not go to zero, so curing will continue at 80oC, but at a much slower rate. Typically one can obtain a Tg about 20-30oC higher than the cure temperature. To investigate this, a sample of the epoxy-amine was cured at 80oC for 6 hours. We chose this time to see how far the cure would advance in a reasonable time. Obviously, in manufacturing one would like to use the shortest cure time possible to get maximum throughput. After the 80oC cure for 6 hours, a sample was placed in the DSC and scanned at 20oC/min to determine the Tg and look for any residual exotherm.
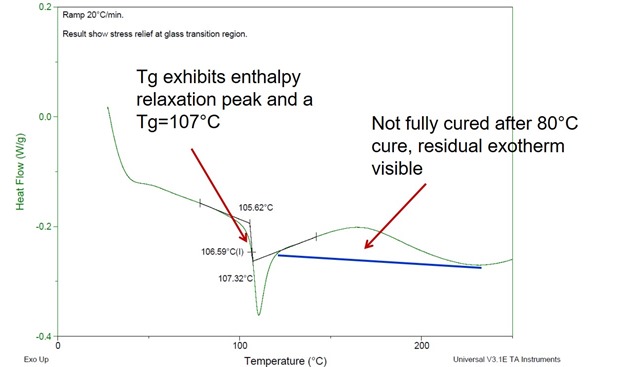
Figure 2. Heat flow as a function of temperature for a two part epoxy amine system after curing for 6 hours at 80oC.
In Figure 2, the Tg after 80oC cure was 107oC. Recall that in Figure 2, the fully cured Tg was 131oC. The sample is not fully cured at 80oC based on the Tg. Additionally, there is residual exotherm visible above the Tg. Remember from our DSC posts, heat is released (exothermic reaction) during curing and above Tg the material has additional segmental mobility and will start to cure as the temperature is increased. The presence of residual exotherm indicates less than full cure. Also, the Tg of 107oC is in the range of the 20-30oC above the cure temperature as we mentioned above.
From a practical standpoint, using an 80oC curing temperature for this epoxy-amine system will result in an under-cured metastable network. If the epoxy-amine system is exposed to temperatures higher than approximately 110oC during use, additional curing can occur and the properties would change.
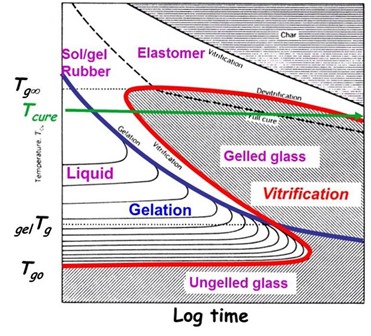
If you look at the Time-Temperature-Transformation (TTT) diagram in the upper left, curing at below the ultimate Tg is represented by the green horizontal line. During isothermal curing, the epoxy-amine system will go through gelation and then when the Tg=Tcure, the system will vitrify with the resulting significant slowing of the reaction rate. In our example the epoxy is under-cured after the 80oC cure process and the Tg is 107oC compared with a fully cured Tg of 131oC.
If you where in charge of this process, how would you increase the Tg?

Leave a Reply