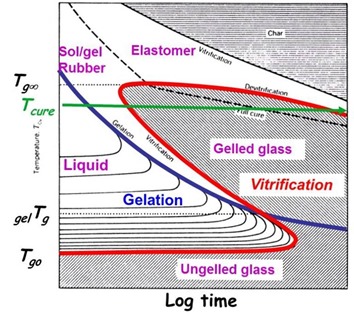
 In our last post we showed an epoxy amine system that was cured at 80oC for six hours resulting in a Tg of 107oC. DSC analysis showed the fully cured Tg was 131oC. What could you do to increase the Tg? Based on the TTT diagram, the best option would be to increase the cure temperature. With increased temperature one could also shorten the curing time. A safe bet would be to increase the cure temperature to at least 130oC to ensure the crosslinking reaction will proceed under chemical control (i.e. reaction temperature is below Tg and thus has higher segmental mobility). At the higher cure temperature the time required to achieve full cure would also be significantly shorter. A way to determine the optimum temperature/time cure profile would be to do a couple combinations of cure temperature and curing time and run the samples or parts in the DSC to measure the Tg. Use the lowest temperature and shortest cure time to get a stable, fully cured Tg.
In our last post we showed an epoxy amine system that was cured at 80oC for six hours resulting in a Tg of 107oC. DSC analysis showed the fully cured Tg was 131oC. What could you do to increase the Tg? Based on the TTT diagram, the best option would be to increase the cure temperature. With increased temperature one could also shorten the curing time. A safe bet would be to increase the cure temperature to at least 130oC to ensure the crosslinking reaction will proceed under chemical control (i.e. reaction temperature is below Tg and thus has higher segmental mobility). At the higher cure temperature the time required to achieve full cure would also be significantly shorter. A way to determine the optimum temperature/time cure profile would be to do a couple combinations of cure temperature and curing time and run the samples or parts in the DSC to measure the Tg. Use the lowest temperature and shortest cure time to get a stable, fully cured Tg.
Now, what happens during room temperature curing? There are many thermoset coatings and adhesives (think about “5 MInute Epoxy”) that are cured at room temperature (or close) in everyday applications. Let’s look at the epoxy amine system from the last post. A sample was cured at room temperature (23-25oC) for 24 hours and run in the DSC.
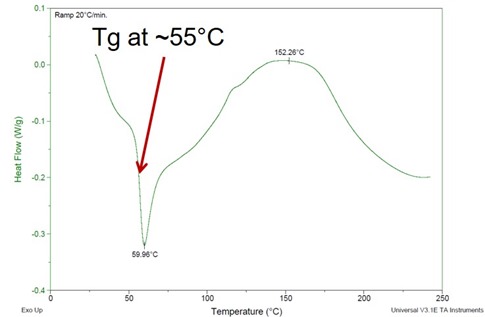
Figure 1. DSC of epoxy amine cured for 24 hours at room temperature (25oC)
In Figure 1, the Tg is approximately 55oC. Two features to note; there is a large enthalpy relaxation peak at 60oC and an exotherm once the temperature is above the Tg indicating further crosslinking. In a previous posts (1, 2) we showed that vitrification occurs when Tg=Tcure. From the DSC, the Tg after room temperature curing was 55oC, approximately 30oC higher than the cure temperature.
Let’s look at another example. In Figure 2, DSC scan for a different epoxy amine curing system is shown after 5 days of curing at room temperature.
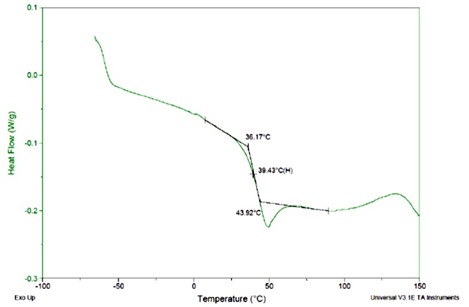
Figure 2. DSC of epoxy amine cured for 5 days at room temperature (25oC)
In this case, the Tg is in the range of 40oC. Two features should be noted: in this system there is a small enthalpy relaxation peak and there is an exotherm after the glass transition indicating additional crosslinking. In this case, the Tg is approximately 15oC above the curing temperature (25oC). Most likely the Tg will slowly increase over time due to continued reaction in the glassy state.
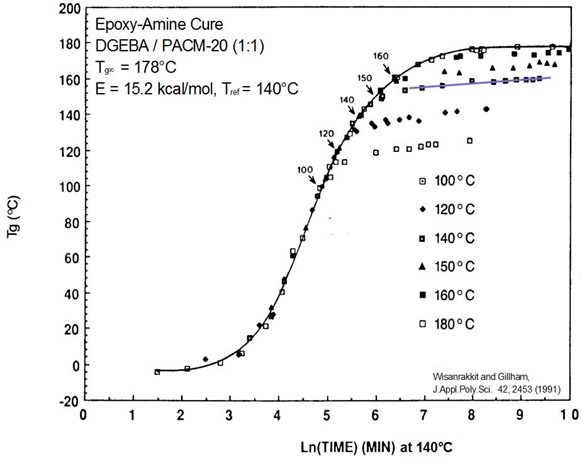
Literature data (2) has clearly demonstrated that when Tg=Tcure, the reaction rate significantly slows, but does not stop. In Figure 2 the glass transition temperature is plotted as a function of time for isothermal curing at 6 temperatures noted in the figure.
Figure 3. Tg as a function of time master curve for an epoxy amine system during isothermal curing at various temperatures.
Let’s look at the data for the 140oC cure temperature. One observes that during the initial curing, all of the Tg-time data fall on the same curve. When the cure temperature reaches 140oC in this example, the Tg continues to increase and then when the Tg gets to be approximately 20oC above the cure temperature there is a very pronounced decrease in the reaction rate. This is highlighted by the solid line in Figure 3. It is clear that the reaction rate significantly decreases, but there is still a positive slope indicating that the Tg continues to increase with curing time, but albeit at a much lower rate.
The glass transition temperature and cure temperature relationship during curing has many practical implications. First is that when choosing a thermoset, careful consideration needs to be given to the desired properties and the implications of the curing process. For many room temperature curing systems, the mechanical and physical properties are sufficient for the application or end use. For higher temperature systems, the choice of cure temperature may be the determining factor.
As a general rule of thumb (and there always is exceptions), the Tg will be in the range of 20-30oC above the cure temperature. In the above cases, the curing was done on a small sample or part. There are exceptions to the 20-30oC rule when the cure exotherm is large and the part heats significantly. Additionally, the Tg can be significantly higher than the cure temperature for UV curing systems. These topics will be the subject of future posts.
References
1. https://polymerinnovationblog.com/thermoset-characterization-part-7-introduction-vitrification/
2. https://polymerinnovationblog.com/thermoset-characterization-part-8-time-temperature-superposition/

Leave a Reply