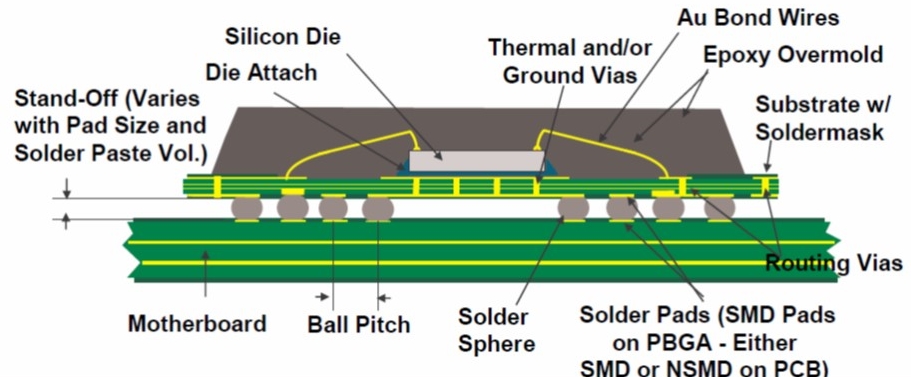
Figure 1. Cross section of PBGA package showing a multilayer semiconductor substrate (Source: NXP Freescale)
A previous post described the plastic ball grid array (PBGA) package. This post will provide an introduction to semiconductor substrate technology. In Figure 1, the semiconductor substrate is directly under the wire-bonded silicon die. In many PBGA’s with relatively low I/O, a double sided substrate is used. The double sided substrate is a BT epoxy core with fan-out circuitry on the top and bottom side and mechanically drilled through holes to connect the top and bottom of the substrate. The first generation of PBGA’s were typically double sided and represent the largest volumes of PBGA’s. Mitsubishi Gas Chemical and Hitachi chemical provide a large selection of copper clad cores used to circuitize the substrates.
The substrate technical drivers are:
- materials with lower CTE, specifically low z-axis expansion
- Low dielectric constant (Dk < 4) and low dissipation factor (Df< 0.01) over a wide frequency band
- Adhesion to low Dk/Df resins
- Thinner cores that enable smaller vias for high density routing
- Low warpage for thin flat substrates
The primary substrate core supplier is Mitsubishi Gas Chemical (MGC) and has supplied the industry with bismaleimide triazine (BT) laminates since the early 1980’s. MGC BT is actually a proprietary blend of bismaleimide, cyanate ester (triazine) and epoxy (US patent 4,110,364). MGC provides a wide range of copper clad laminates (CCL) and prepregs. The CCL HL832NX (laminate) and GHPL-830NX (prepreg) are the standard halogen-free BT with a flammability rating of UL94V-0. Click for a complete listing of the MGC BT product offerings.
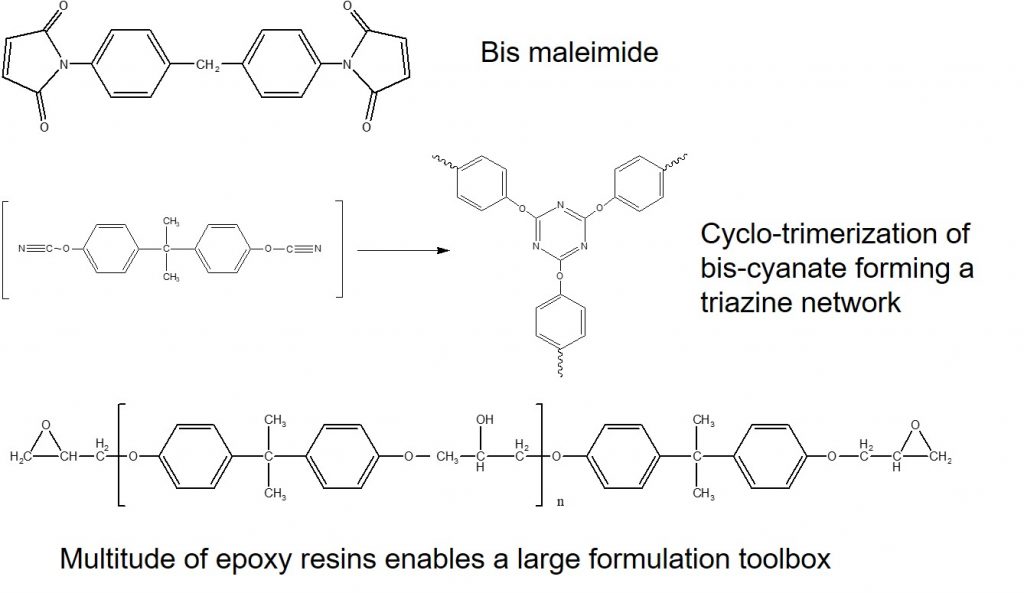
The chemistry of the bismaleimide triazine (BT) resin is shown in Figure 2.
Figure 2. Chemical components of MGC BT resin systems.
In Figure 2, the chemical structures are given for the three main components of BT laminates are prepregs. Owing to the high glass transition temperatures for the fully cured BT laminates, aromatic bismaleimides were used as shown in the top image. The MGC patent describes a large variety of bismaleimides that can be used. Cyanate esters are interesting in that they have very low dielectric constant and more importantly have a low dielectric loss factor. The cyanate functionality undergoes a cyclotrimerization reaction leading to a triazine ring structure. The most common cyanate ester is synthesized from bisphenol A to form the aromatic monomer. After trimerization, a very high Tg is obtained.
The third component of the BT resin system is epoxy. Epoxy monomers are available in various monomeric forms, from liquids epoxies, to novolac epoxies, to the common Bis-A epoxy (as shown in the bottom in Figure 2). Epoxies are also available in several molecular weights. The curing package for epoxies is typically diamines. It turns out that diamines are also useful curing agents for the bismaleimides via the Michael addition reaction to the bismaleimide double bonds.
The MGC patent also describes various amines that can be useful in tailoring the physical properties of the BT resin system. In the ‘364 patent, example 11 shows a typical BT formulation with 10 parts by weight bismaleimide, 20 parts by weight cyanate ester and 70 parts by weight epoxy (DER 542) and 3 parts by weight of an amine curing agent, 2-ethyl-4-methylimidazole (24EMI).
Interesting personal note: When I was a very new Ph.D. at IBM, I was assigned to develop new dielectric materials for large mainframe circuit boards. Our goal was to decrease the dielectric constant and loss factor to enable faster mainframe computations. One of the first suppliers to meet with me in 1983 was Mitsubishi Gas Chemical. I got to meet Morio Gaku, the main inventor on the BT patent (4,110,364, issues in 1978) and somewhat of a legend in the circuit board and substrate industry. We were looking at evaluating MGC BT resins or working together. He was very cordial and open about the MGC approach. I still remember our meeting to this date.

Jeffrey,
What comments can you make about the volatility and/or relative importance to subsequent assembly silicone adhesive curing of the following?
“…typical BT formulation with 10 parts by weight bismaleimide, 20 parts by weight cyanate ester and 70 parts by weight epoxy (DER 542) and 3 parts by weight of an amine curing agent, 2-ethyl-4-methylimidazole (24EMI)”
Is it ever the case that these BT ingredients out-gas during subsequent assembly, and possibly impede adhesive curing?
Jim Bustillo