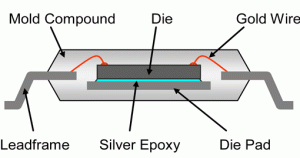
 The last post introduced the basics for die attach adhesives. This post will provide more details into the chemistry of die attach adhesives over time. The one of the first uses of die attach adhesives was to attach the semiconductor chip (or die) to a leadframe substrate as seen in the schematic cross-section on the left.
The last post introduced the basics for die attach adhesives. This post will provide more details into the chemistry of die attach adhesives over time. The one of the first uses of die attach adhesives was to attach the semiconductor chip (or die) to a leadframe substrate as seen in the schematic cross-section on the left.
The following chart depicts the progression of die attach materials.
The first generation of paste die attach adhesives used in the 70’s and 80’s was epoxy based and provided a good combination of cost and performance. There are still a large number of epoxy-based paste die attach adhesives on the market today. The term “paste” die attach is used to characterize adhesives that are composed of liquid resins and fillers, typically silver or silica. Paste die attach adhesives are provided in syringes and dispensed using a variety of methods.
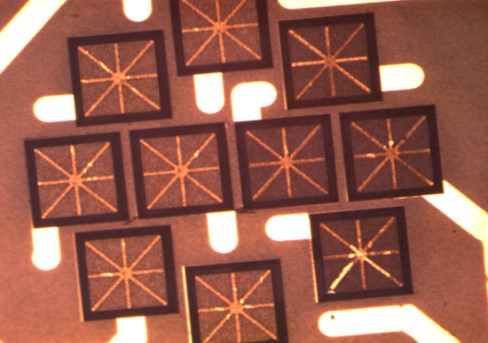
The silver flakes in the die attach paste are in the range of 2-10 microns and are surface treated to improve the rheology and electrical conductivity (1). The silver flakes impart an important rheological property of shear thinning. Under shear, the viscosity drops dramatically allowing the epoxy-based, silver filled adhesive to flow onto the leadframe or BGA substrate. Once the die attach paste is on the surface, the silver-silver attraction causes the viscosity to increase quickly resulting in a clean, non-slumping dispense pattern as seen in the photo above. The “askerisk” or “snowflake” pattern is used to ensure uniform flow to the die edges allowing formation of a uniform fillet between the die side and the substrate.
The main improvement in die attach technology occurred in the early 1980’s when clean epoxies were developed. The ultra-clean epoxies significantly reduced reliability failures from ionic contamination migration and corrosion. One of the pioneering die attach adhesives to use ultra-clean epoxy was Ablestik 84-1 LMI (low mobile ions) and it still is in wide use today. The drawback of epoxy die attach adhesives is the relatively slow curing profiles. From the TDS for 84-1 LMI, but recommended curing is 1 hour at 150°C which is pretty slow for today’s high speed packaging lines. The second generation die attach adhesives had low outgassing, low resin bleed, and had good electrical conductivity through the use of silver flakes
In the early 1990’s the plastic ball grid array (PBGA) package was developed and required a non-conductive die attach with faster curing. In the late 1990’s the initiative to remove lead from electronic packages and the transition to lead-free packages resulted in a shift in resin chemistries:
- Thermosetting resins with improved adhesion to pre-plated leadframes (PPF) having a thin gold surface
- Lower moisture absorption (hydrophobic resins)
- Higher temperature stability (lead-free reflow at 260°C)
- Lower stress after curing (warpage control as the die size increases)
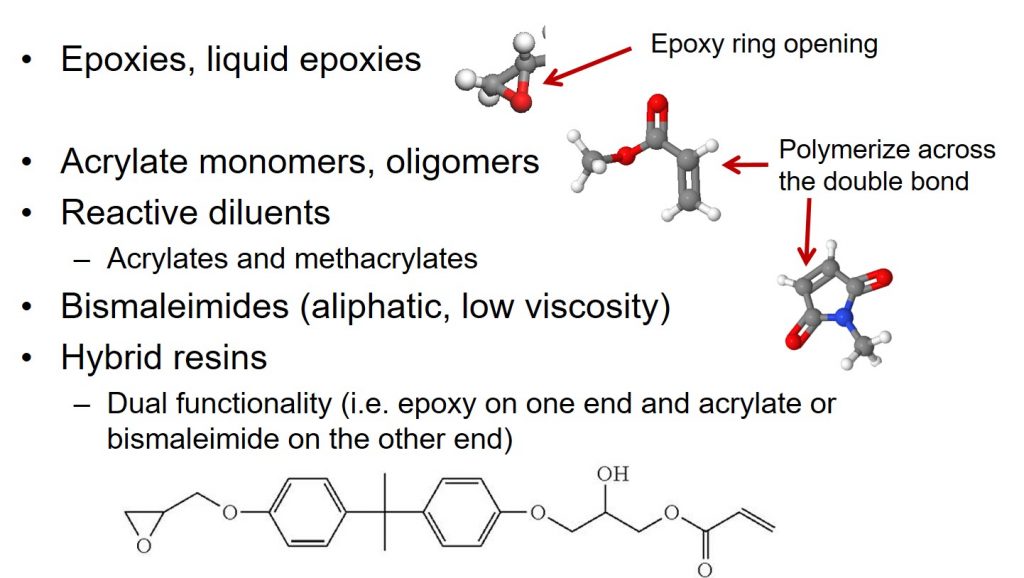
Faster cure times a lower cure temperatures required the introduction of acrylates and maleimides that could be cured using free radical initiators. Very fast curing systems were developed using peroxides. Additionally, the third generation of die attach adhesives had to adhere to gold surfaces with the advent of the pre-plated leadframe (PPF). PPF leadframes have a thin plated gold surface.
The following figure summarized the progression of resin chemistries used in die attach adhesives.
The next post will cover the introduction of bismaleimides for fast-curing, low moisture absorption die attach adhesives for BGA packages.
Reference:
1) Die Attach Adhesives and Films, Shinji Takeda and Takashi Masuko, Chapter 12, Materials for Advanced Packaging, Edited by C. P. Wong and Daniel Lu, Springer, 2009


Dear Dr. Jeff, how then do we remove the acrylates/maleimides type of die attach? I found the removal methods of epoxy type die attach: https://www.c3teknoloji.com/Eklenti/169%2Creworking-removing-and-decapsulating-of-cured-epoxypdfcfcd.pdf?0 Are there any book references for this?