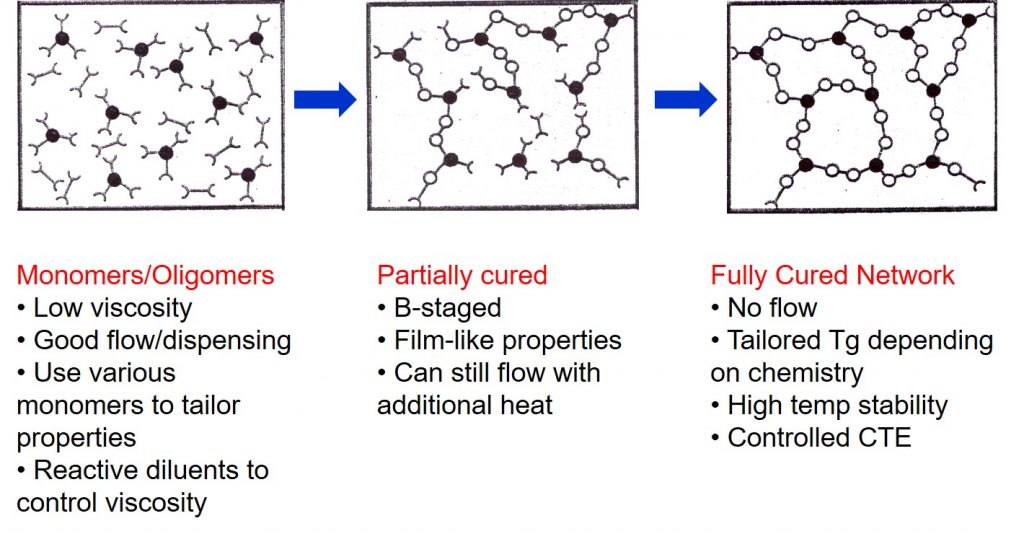
 Thermosets are unique in that during processing a chemical reaction (curing) transforms small molecules into a large crosslinked network as depicted in the schematic on the left. The use of small molecules as starting materials allows for many types of processing such as adhesive dispense, flip chip underfill, molding of epoxy mold compounds, application of protective coating layers, lamination of multilayer printed circuit substrates and more. Typical thermoset resins used in electronics are epoxies, phenolics, acrylates and methacrylates, bismaleimides, and cyanate esters. During curing low viscosity monomers and oligomers undergo a chemical reaction that changes the thermoset from a low viscosity liquid to a crosslinked network structure.
Thermosets are unique in that during processing a chemical reaction (curing) transforms small molecules into a large crosslinked network as depicted in the schematic on the left. The use of small molecules as starting materials allows for many types of processing such as adhesive dispense, flip chip underfill, molding of epoxy mold compounds, application of protective coating layers, lamination of multilayer printed circuit substrates and more. Typical thermoset resins used in electronics are epoxies, phenolics, acrylates and methacrylates, bismaleimides, and cyanate esters. During curing low viscosity monomers and oligomers undergo a chemical reaction that changes the thermoset from a low viscosity liquid to a crosslinked network structure.
For the most part, thermosets are used for a number of reasons:
- Low viscosity in the uncured state to allow flow
- Wide range of cure profiles
- Thermal (oven, snap and spot cure) and UV cure
- Partial cure (B-stage) for printable pastes and films
- Tailored modulus depending on the application
- Low coefficient of thermal expansion
- High temperature stability for lead-free reflow profiles
- Low moisture absorption
- Suitable for use with fillers (conductive and non-conductive)
In most electronic applications thermoset resins are formulated with high performance fillers such as silver (electrically conductive adhesives), silica (electrically insulating adhesives and epoxy mold compounds), rheology modifiers, reactive diluents, and toughening agents. These highly formulated liquids can be processed from the liquid state to a fully cured network as shown in the following figure.
The initial formulation is typically a low viscosity liquid that can be dispensed using a variety of methods. Careful choice of the monomers and oligomers is used to tailor the starting viscosity. The addition of fillers increases the viscosity. For common die attach pastes for example, the high silver flake loading requires the use of reactive diluents to aid in achieving a sufficiently low viscosity for dispensing. Typical thermoset formulations are mixtures of various types of monomers to give the desired final properties in the fully cured network. Materials in the uncured state are called A-staged.
An interesting feature of thermosets is that you can partially react/cure to what is called the B-stage. The chemical reactions cause the molecular weight to increase, typically resulting in chain extension. If the reaction is stopped prior to the gel point, the material will be able to flow and resume curing on the application of additional heat. The advantage of B-staging is that the intermediate level of cure provides a material with film-like properties. B-staging is usually done at a temperature lower than the final cure temperature, or the time at an elevated temperature is carefully controlled to reach the desired level of conversion.
A good example is die attach film used extensively in multi-die stacking. The film die attach formulation is coated onto a carrier and partially cured so it does not have too much flow, but is just tacky enough to easily bond during the pick and place operation.
The final step in the processing of thermosets is to achieve a fully cured network. Many of the adhesives used in electronics are high Tg materials, and need to be cured above 100°C to get the desired properties. The final curing results in the network achieving the desired Tg. Since the fully cured network will not flow, for example during solder reflow, the adhesive or coating will have high temperature stability. Choice of suitable resins and fillers will allow the coefficient of thermal expansion (CTE) to be tailored for the particular application.
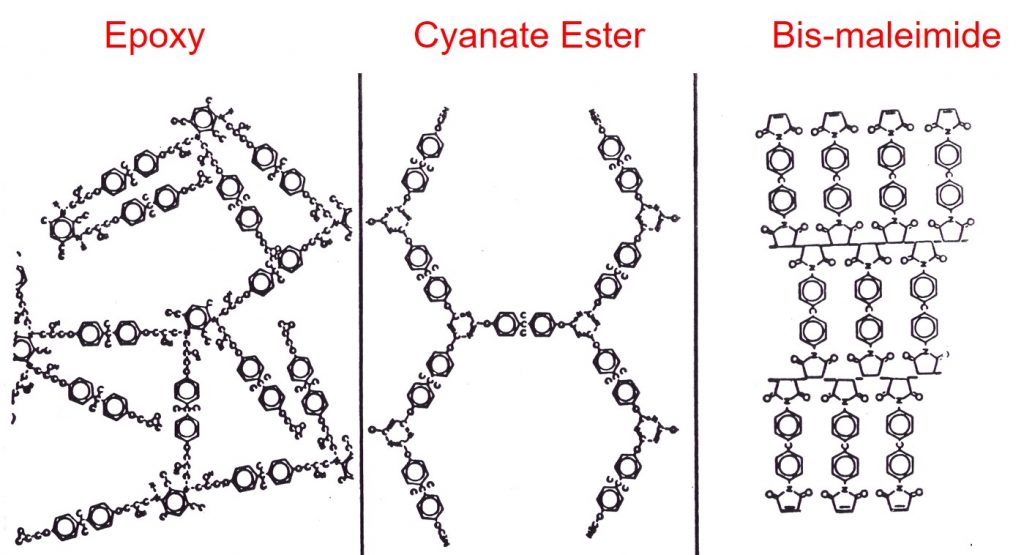
The fully cured network can have many structures depending on the starting monomers and curing agents. In the following figure, three different schematics of thermoset networks are shown.
The epoxy networks are typically formed via the reaction of epoxies and diamines and have a random orientation as shown on the left. Bisphenol A dicyanate esters crosslink via a cyclotrimerization reaction leading to a highly aromatic network structure as shown in the center schematic. Unmodified bismaleimides crosslink through the maleimide double bond leading to an alternating type of network. Each one of these networks has unique properties in terms of Tg, modulus, moisture absorption, and fracture toughness.

Leave a Reply