 This post will provide an overview of the thermoset resins used as the polymer matrix in typical composites. The choice of the resin matrix is important in that it defines some of the critical composite properties such as toughness, moisture absorption, chemical resistance, weathering, permeability, compatibility and fiber wet-out, along with thermal and electrical properties. The resin is chosen to meet the use conditions. Lower end fiber reinforced plastics (FRP’s) are mainly used for room temperature or moderate use environments such as shower enclosures, sporting goods, boat hulls, decorative panels and automotive applications. Advanced composites are typically used at higher temperatures and harsher operation conditions such as aerospace, high temperature automotive applications, high-end bicycles, wind turbine blades, and structural composites (requiring high modulus).
This post will provide an overview of the thermoset resins used as the polymer matrix in typical composites. The choice of the resin matrix is important in that it defines some of the critical composite properties such as toughness, moisture absorption, chemical resistance, weathering, permeability, compatibility and fiber wet-out, along with thermal and electrical properties. The resin is chosen to meet the use conditions. Lower end fiber reinforced plastics (FRP’s) are mainly used for room temperature or moderate use environments such as shower enclosures, sporting goods, boat hulls, decorative panels and automotive applications. Advanced composites are typically used at higher temperatures and harsher operation conditions such as aerospace, high temperature automotive applications, high-end bicycles, wind turbine blades, and structural composites (requiring high modulus).
Unsaturated Polyester Resins
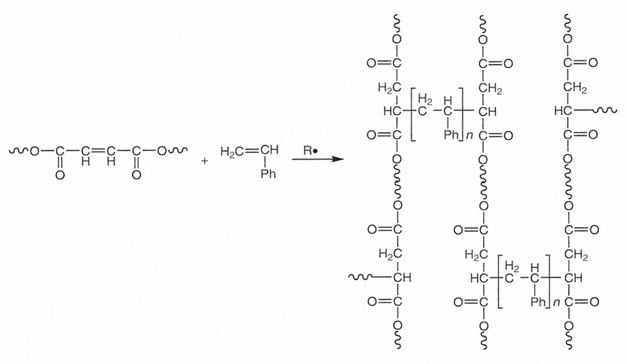
Unsaturated polyester resins are the most common matrix due to their low cost and ease of use in many applications. Typical unsaturated polyester resins are co-cured with styrene monomers using a free radical initiator. There are many types of free radical initiators such as peroxides and azobisisobutyronitrile (AIBN) in the formulators toolbox. The double bonds on the polyester backbone as well as the pendent double bond on the styrene monomer provide pathways for both chain extension and crosslinking as shown in the following schematic (ph = aromatic six member ring):
Epoxy Resins
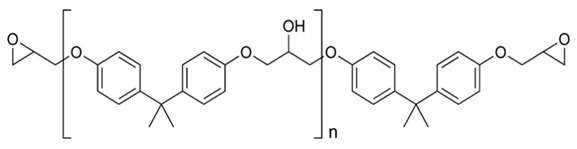
Epoxy resins are a well established technology, are the second most widely used thermoset, and offer a good cost-performance benefit. A large amount of epoxy resins are used in what most call non-reinforced applications such as adhesives, paints, coatings, but many of these applications have fillers and other modifiers to enhance the end-use properties. There is an extensive toolbox of epoxy resins (bisphenol A epoxy, cycloaliphatic epoxy, epoxy novolac, and epoxy cresol novolac) along with hardeners and catalysts. Epoxies can be cured at room temperature as well as at high temperatures for high Tg epoxy composites. The most common epoxy monomer is the diglycidyl ether of bisphenol A epoxy:
Vinyl Esters
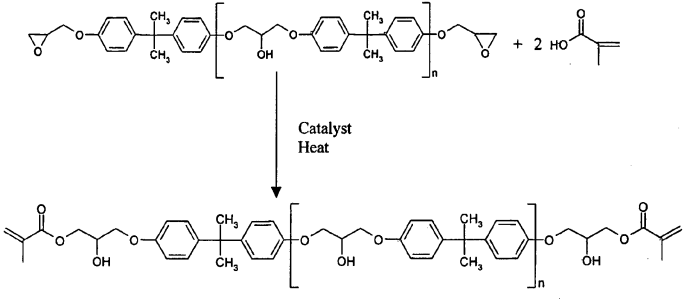
Vinyl esters are an interesting type of thermoset in that they are derived from an epoxy resin but due to the pendant double bond at the end of each monomer, they react and are cured using a free radical initiator like in the case of unsaturated polyesters. The modification of the epoxy resin is achieved through the reaction of the epoxy group with an acrylate. In this case, the epoxy functional group is changed to an acrylic (double bond) end group. The reaction pathway is shown below for the modification of a bisphenol A epoxy resin:
Vinyl esters can be made using a variety of epoxy monomers, thus there are Bis A and epoxy novolac vinyl ester resins available. Interestingly, the performance of vinyl esters is in between epoxies and unsaturated polyesters. The vinyl esters have many of the advantageous properties of epoxies but also have good toughness, thermal and electrical stability, low cure shrinkage and low volatiles during manufacturing.
Bismaleimide Resins
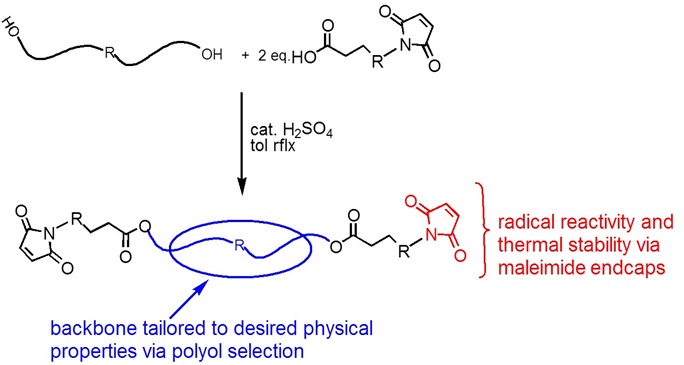
Bismaleimide resins are a broad class of both aromatic and aliphatic monomers in the polyimide family. These are termed addition polyimides since they react via an addition reaction across the maleimide double bond. Due to the bismaleimide synthetic pathway, many types of bismaleimides can be synthesized:
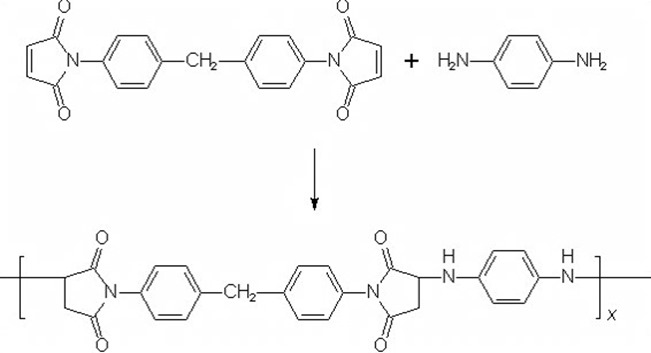
The polyol can either be aliphatic if a flexible, low Tg network is desired (in adhesive applications) or a highly aromatic polyol can be used to form a very stiff, high Tg network for use in high performance applications like aerospace composites. An example of an aromatic bismaleimide cured via Michael addition using an aromatic amine is shown below:
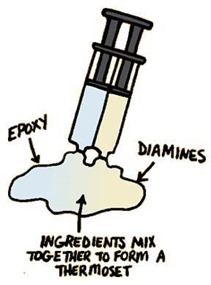
The resulting network structure will be very rigid and have a high glass transition temperature and high stiffness. The use of diamines are used to modify maleimides by chain extension to lower the steric hindrance allowing higher degree of polymerization. Epoxies can be added in increase toughness and will co-react with the diamines leading to a flexible formulation toolbox.
Cyanate Ester Resins
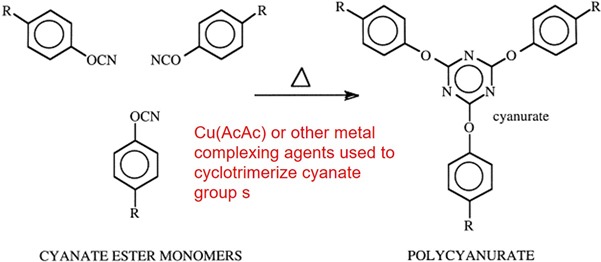
Cyanate esters are used where high Tg and low dielectric loss is needed. Cyanate esters react via a cyclotrimerization reaction using a coordination type catalyst (bring the three cyanate groups into close proximity):
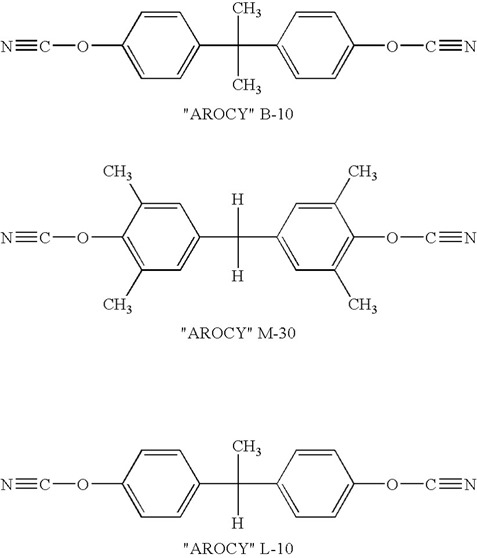
The most common cyanate ester monomer is the bisphenol A dicyanate formed by functionalizing the phenolic OH group with the cyanate group. A family of cyanate esters is available analogous to the various types of epoxies derived from bishpenol A starting materials.
The highly crosslinked bis A dicyanates have a very high Tg, approaching 300oC when fully cured, but suffer from brittleness and low toughness. Typically, epoxy is added to increase the toughness (with a decrease in the Tg) or the addition of the second phase thermoplastic toughener is also used.

Leave a Reply