Guest blog post by Jeremy Pasatta, Advanced Polymer Coatings
In industrial protective and marine coatings, having a smooth uniform finish is critical to performance attributes such as corrosion protection, discharge of viscous cargoes and cleanability for internal coatings. One crucial component in ensuring this is the use of wetting agents. These additives play a vital role in optimizing the performance and appearance of coatings. In this blog post, we’ll look into the chemistry behind wetting agents, exploring their mechanisms and how they contribute to coating effectiveness.
Wetting agents, also known as surfactants or surface-active agents, are compounds that reduce the surface tension at an interface of two materials. For coatings, these additives typically lower the surface tension between the coating and the substrate improving the liquid’s ability to spread and adhere uniformly. Addition levels are typically very low, not exceeding more than a few percent of the total formulation weight.
Wetting agents are characterized by their amphiphilic nature—meaning they have both hydrophilic (water-attracting) and hydrophobic (water-repellent) parts. This dual nature allows them to interact simultaneously with both polar and non-polar components, enhancing their ability to penetrate and spread across surfaces.
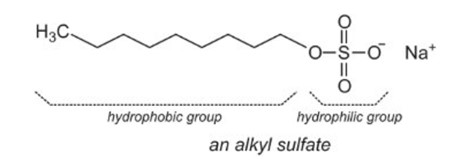
The most common structure of a wetting agent consists of a hydrophobic tail and a hydrophilic head. The hydrophobic tail is usually a long hydrocarbon chain, while the hydrophilic head is often a polar or ionic group. This structure enables the wetting agent to adsorb at the interface between water and oil or air, reducing surface tension and improving spreading. A representative amphiphilic surfactant is an alkyl sulfonates, which is shown in Figure 1.
Figure 1. Structure of an Alkyl Sulfonate Surfactant [1].
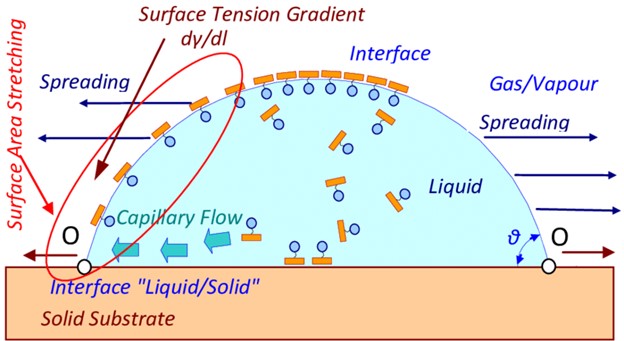
Wetting agents work by aligning themselves at the interface between different phases. When added to a coating formulation, they create a surface tension gradient across the surface of the liquid, which in turn creates a capillary flow leading to a lower contact angle of the liquid and increased spreading. This general mechanism is shown in Figure 2.
Figure 2. Spreading Mechanism of a Coating Containing a Wetting Agent [2].
Wetting agents can be classified into several categories based on their chemical structure and properties. In addition to the structures below, other types of wetting agents include siloxane-based molecules, perfluorinated based molecules and block copolymers.
- Anionic Surfactants: These have a negatively charged hydrophilic head and are effective in reducing surface tension in both acidic and alkaline environments.
- Cationic Surfactants: Featuring a positively charged hydrophilic head, these surfactants are often used in formulations requiring antimicrobial properties.
- Nonionic Surfactants: These do not carry a charge and are often used in a wide range of pH environments, providing excellent wetting properties.
- Zwitterionic Surfactants: These possess both positive and negative charges and are used in specific applications requiring minimal irritation.
Wetting agents are integral to the formulation of coatings for several reasons:
- Improved Spreadability: By lowering surface tension, wetting agents allow coatings to spread more evenly over surfaces, minimizing issues like orange peel or uneven gloss.
- Enhanced Adhesion: Better wetting translates to improved adhesion of the coating to the substrate, which can lead to greater durability and longevity of the finish. The amount of wetting agent should be kept to a minimum though in base coats, because too high of a concentration of wetting agent can lead to poor adhesion of the topcoat to the basecoat.
- Reduced Defects: Proper wetting helps in avoiding common defects such as bubbling, pinholes, and poor leveling, ensuring a smoother final appearance. This allows coatings to be applied at lower thickness with fewer defects. A high number of defects can affect the overall corrosion and chemical resistance of the coating
- Compatibility: Wetting agents can enhance the compatibility of different components in a coating formulation such as epoxy and inorganic fillers, leading to more stable and uniform coatings.
- Enhanced Cleanability and Cargo Discharge: For internal tank linings a smooth surface allows for rapid discharge of viscous oily cargoes with the use of less heating, resulting in more efficient operation. A smooth surface also allows the coating to be cleaned more effectively, again leading to a more efficient operation.
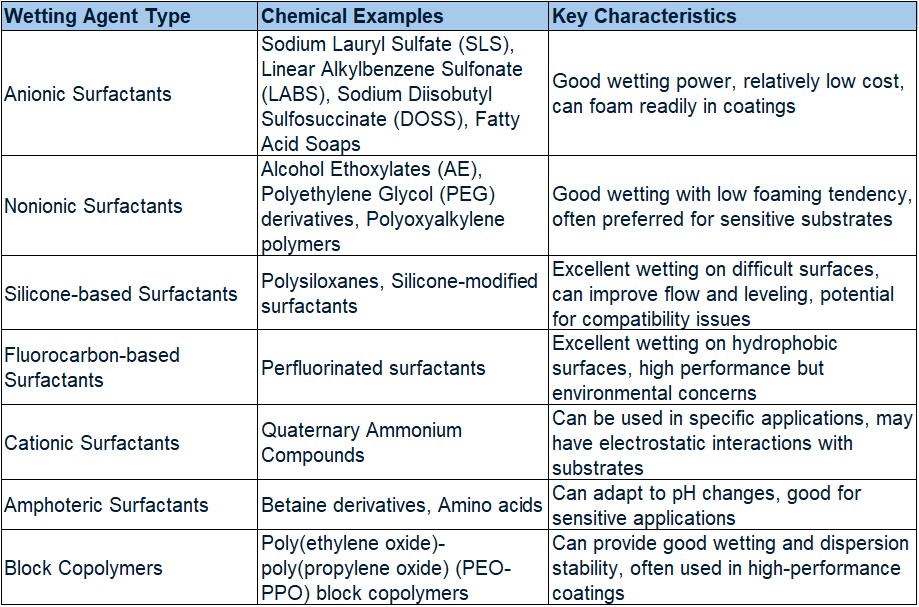
Table 1 lists the most common types of wetting agents used in coatings along with their positive and negative attributes.
Table 1. Common Wetting Agents Used in Coatings
The effect of a wetting agent in a two-component epoxy-based coating to prevent cratering is shown in Figure 3. This figure shows that only a very low addition to a coating is necessary to resolve surface defects.
Figure 3. Elimination of Cratering in a 2K Epoxy Coating Using a Wetting Agent [3].
In this blog post we have seen that wetting agents are essential for the successful application of coatings, ensuring that they spread evenly and adhere properly to various surfaces. Their chemistry, characterized by a combination of hydrophilic and hydrophobic regions, allows them to lower surface tension and improve performance. Other types of wetting agents, such as siloxanes, perfluorinated molecules, and block copolymer can also effectively reduce the surface tension of the coating and promote wetting. By leveraging the chemistry of wetting agents, formulators can address common issues in coating applications, leading to higher-quality finishes, more reliable coatings and coatings that improved operational efficiencies.
References:
- https://www.essentialchemicalindustry.org/materials-and-applications/surfactants.html
- Dineff, Peter & Ivanov, Ivaylo & Gospodinova, Dilyana. (2014). Plasma-aided capillary impregnation for flame retardancy of wood II. Silicone surfacе-active agent effect. Proceedings of Technical University of Sofia. 64. 87-96.
- https://coatings.specialchem.com/selection-guide/wetting-agents#:~:text=They%20are%20very%20efficient%20on,are%20emerging%20in%20this%20category.


Leave a Reply